CDSCO License for Percutaneous Catheter
Medical Device Information
Intended Use
A needle catheter getting access to a blood vessel, followed by the introduction of a wire through the lumen (pathway) of the needle.
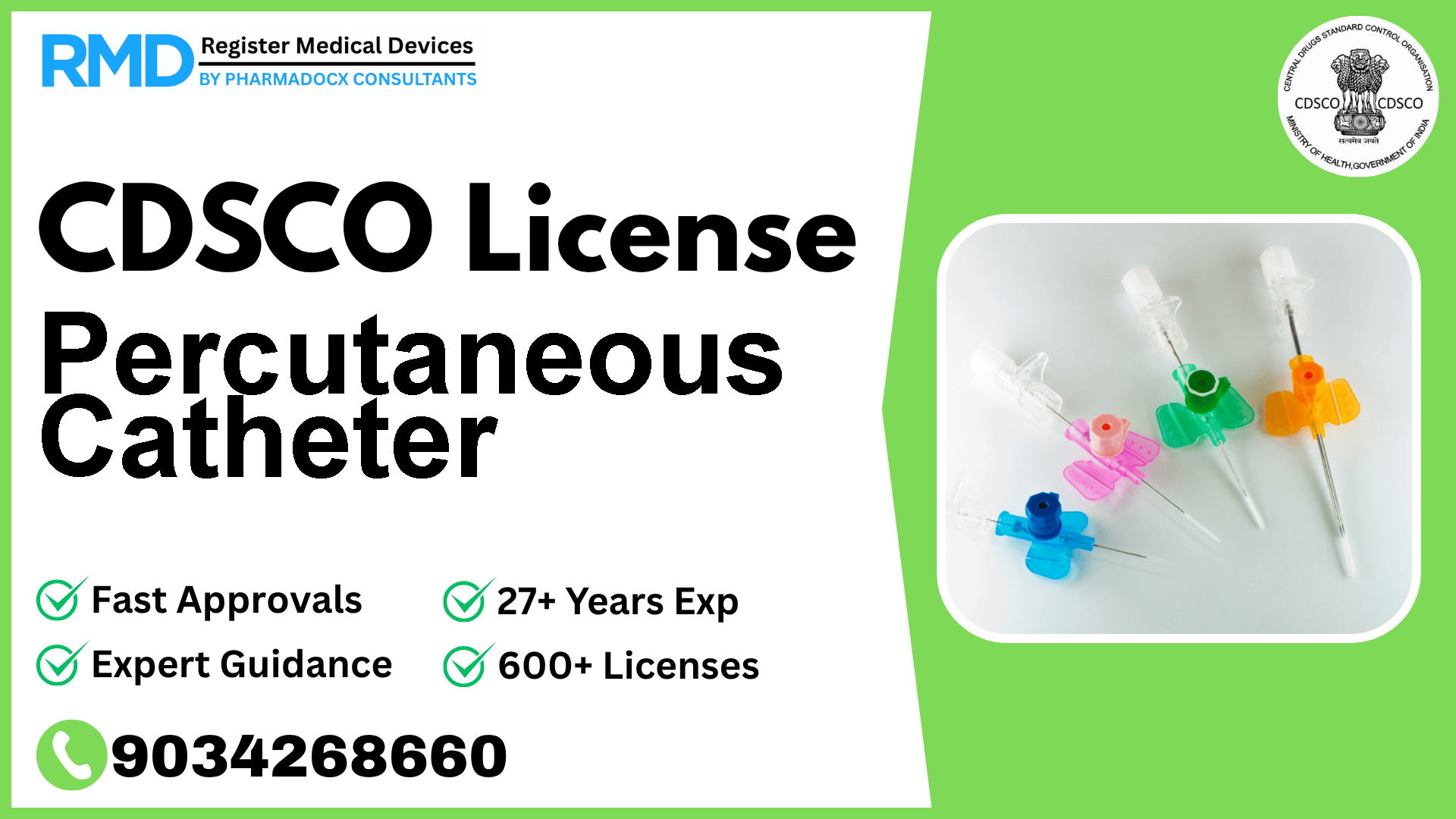
Comprehensive Guide to CDSCO Licensing for Percutaneous Catheters (Class D Medical Devices)
As seasoned regulatory consultants with over 25 years of experience and having assisted more than 500 companies in securing CDSCO licenses, we understand the complexities manufacturers and importers face when bringing advanced medical devices like Percutaneous Catheters to the Indian market. These devices, classified as Class D due to their critical role in vascular access, require stringent regulatory compliance under the Central Drugs Standard Control Organization (CDSCO) framework.
Introduction to Percutaneous Catheters and Regulatory Significance
A Percutaneous Catheter is a specialized needle catheter designed to gain vascular access by introducing a guidewire through the needle’s lumen. Given its direct interaction with blood vessels and potential risk of severe patient harm, it is classified as a high-risk Class D device as per CDSCO’s notification 29/Misc/3/2017-DC (292) dated 06.06.2018. Regulatory approval ensures the highest standards of safety, efficacy, and quality before market entry.
CDSCO Regulatory Framework for Percutaneous Catheters
India’s regulatory system for medical devices is risk-based and overseen by CDSCO. Class D devices like Percutaneous Catheters require manufacturing licenses granted by the Central Licensing Authority under Form MD9 (application Form MD7). Importers must obtain an import license on Form MD15 to legally bring these devices into India.
Understanding the regulatory nuances and timelines is critical to streamline approvals while minimizing delays.
Risk Classification and License Requirements for Percutaneous Catheters
- Risk Class: D (Highest risk category)
- Regulatory Notification: 29/Misc/3/2017-DC (292)
- License Type: MD9 Manufacturing License (Central Authority)
- Application Forms: MD7 for manufacturing; MD15 for import
Given the Class D status, CDSCO mandates rigorous documentation, testing, and audits to ensure compliance with Indian and international standards.
Manufacturing License Process for Class D Devices (MD9 License)
The MD9 license process typically spans 4 to 5 months and involves multiple steps:
- Test License (Form MD13): Obtain a test license first, which takes approximately 1.5 to 2 months. This permits testing product samples in government-approved labs.
- Product Testing: Conduct mandatory product testing in CDSCO-recognized laboratories to verify safety and performance.
- Document Preparation: Compile comprehensive technical files including Device Master File (DMF), Plant Master File (PMF), Essential Principles Checklist, Risk Management File, and Quality Management System documents.
- License Application (Form MD7): Submit the manufacturing license application through the CDSCO MD Online Portal.
- Audit and Inspection: CDSCO inspectors perform a detailed audit of manufacturing premises and quality systems.
- Query Resolution: Address any queries or deficiencies raised by the department or auditors promptly.
- License Grant: Upon satisfaction, CDSCO issues the MD9 license (Form MD9), authorizing manufacture.
For detailed guidance, our MD9 License Guide provides step-by-step insights.
Manufacturing License Documents Required for Percutaneous Catheters
Documentation is critical for approval. Key documents include:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Technical Staff Qualification and Experience Records
- Fire No-Objection Certificate (NOC)
- Pollution Control Board NOC
- Device Master File (DMF): Detailed device design, specifications, and risk analysis (refer our DMF guide)
- Plant Master File (PMF): Manufacturing processes, equipment, and quality control (read more)
- Essential Principles Checklist: Compliance with Indian and international standards
- Risk Management File: Hazard analysis, mitigation strategies (best practices here)
- Test Reports: From CDSCO-approved laboratories (list here)
- Labels and Instructions for Use (IFU)
- Quality Management System (QMS) Documents: ISO 13485 certification and SOPs
Import License Process for Percutaneous Catheters (MD15 License)
Importers must apply for an MD15 license, which is granted by the Central Licensing Authority and takes approximately 5 to 6 months.
The process includes:
- Preparation of required documents
- Application submission on CDSCO MD Online Portal
- Resolution of any department queries
- Final license issuance (Form MD15)
Unlike manufacturing, no test license is required for imports, but documentation must demonstrate compliance and approvals from recognized regulatory bodies.
Import License Documents Required
- Valid manufacturing license of the product’s country of origin
- Free Sale Certificate from the country of origin
- ISO 13485:2016 certification
- CE Certificate or equivalent regulatory approval
- Device Master File and Plant Master File
- Wholesale Drug License (if applicable)
- Company Constitution
Timeline and Processing Duration Summary
| License Type | Step | Duration |
|---|---|---|
| Test License | Form MD13 Application | 1.5–2 months |
| Product Testing | Testing in Govt Approved Labs | 1–2 months |
| Manufacturing License | Form MD7 Application (MD9) Audit & Approval | 2–3 months |
| Import License | Form MD14 Application (MD15) | 5–6 months |
Manufacturers should plan for at least 4-5 months for the entire manufacturing license process, considering audits and query resolution.
Government Fees and Costs Breakdown
MD9 Manufacturing License:
- Application Fee: ₹50,000
- Per Product Fee: ₹1,000
Test License (MD13): Included in overall process
MD15 Import License:
- Class D Device Fee (per site): $3,000
- Per Product Fee: $1,500
Note: Fees are payable online via the CDSCO portal and can vary slightly based on amendments.
Common Challenges Faced and Practical Solutions
- Delayed Testing Reports: To avoid bottlenecks, schedule testing early with CDSCO-accredited labs and track progress regularly.
- Incomplete Documentation: Use comprehensive checklists and consult experienced regulatory experts to prepare technical files, avoiding back-and-forth with CDSCO.
- Audit Non-Compliance: Conduct internal mock audits before CDSCO inspection to ensure all QMS and premises meet standards.
- Query Resolution Delays: Maintain clear, prompt communication with CDSCO and prepare detailed responses supported with evidence.
Expert Consultation and Support
Navigating CDSCO’s regulatory landscape for Class D devices like Percutaneous Catheters requires expertise. We offer tailored consulting services, including:
- Gap analysis of technical documentation
- Audit readiness assessments
- Application preparation and submission
- Liaison with CDSCO officials
Our proven track record ensures your application progresses smoothly toward approval.
Getting Started with Your CDSCO License Application
- Assess your Device Classification: Confirm Percutaneous Catheter’s Class D status (Check classification here).
- Prepare Technical Documentation: Develop DMF, PMF, risk management, and QMS documentation as per CDSCO and international standards.
- Apply for Test License (MD13): Submit via the CDSCO MD Online Portal.
- Schedule Product Testing: Engage with government-approved labs early.
- Compile Manufacturing License Application (MD7): Ensure all documents and test reports are complete.
- Plan for Audit: Coordinate with notified bodies or CDSCO inspectors for site inspection.
- File Import License Application (MD15): Once manufacturing license is secured or for direct import purposes.
Starting early with thorough preparation can save months of delays and costs.
Our team is ready to guide you through every stage to ensure your Percutaneous Catheter complies fully with CDSCO requirements and reaches the Indian healthcare market efficiently.
For a tailored consultation or to learn more about our services, contact us today.