CDSCO License for Bile duct brachytherapy system applicator, remote-after loading
Medical Device Information
Intended Use
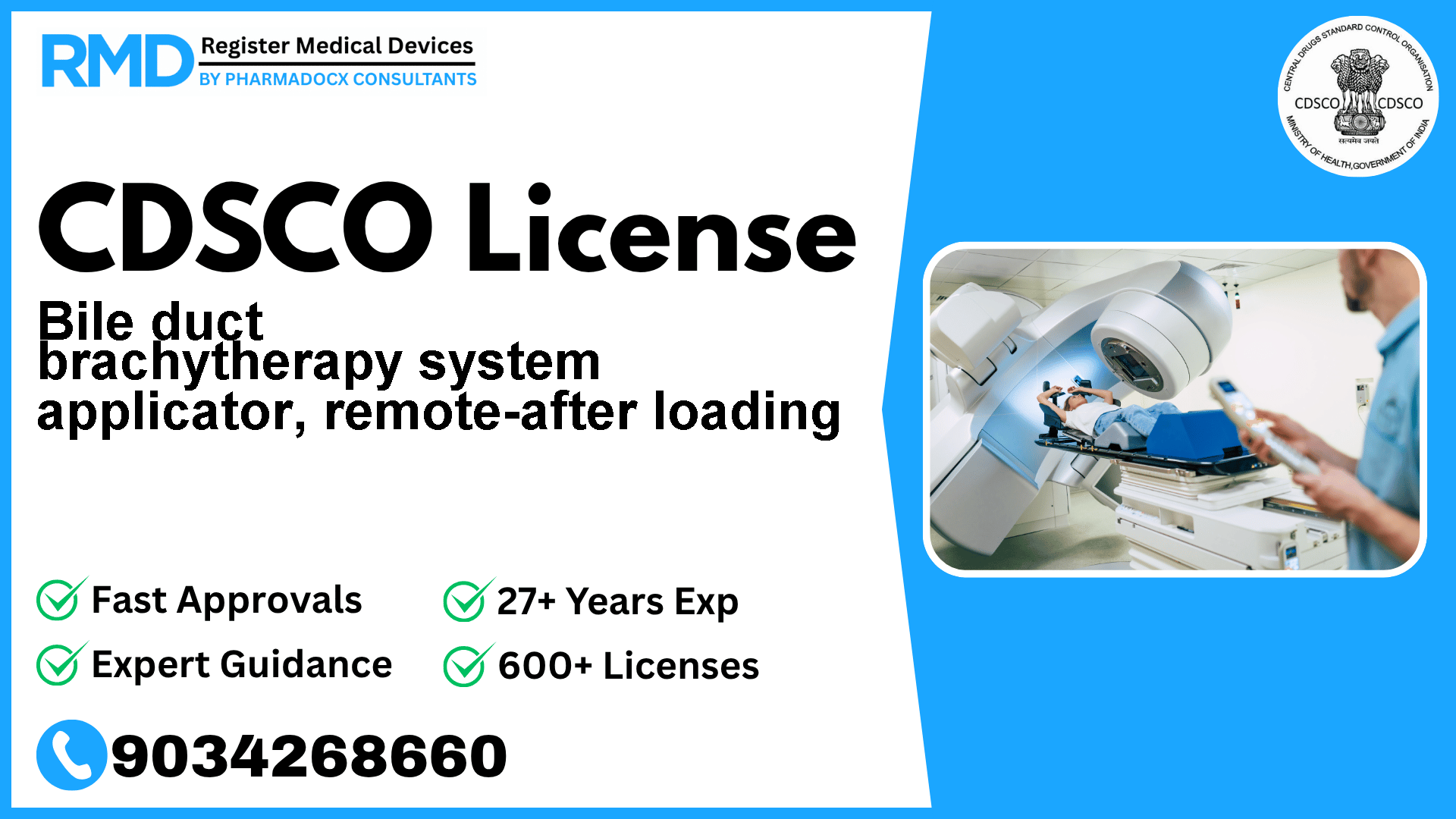
A remote after loading brachytherapy applicator specifically designed for use in radiation therapy treatments of the bile duct. It is designed for temporary insertion into the bile duct and serve as a guide for computer-controlled placement and removal of single or multiple radioactive sources.
Comprehensive Guide to CDSCO Licensing for Bile Duct Brachytherapy System Applicator (Remote-After Loading)
Entering the Indian medical device market with a Class C device such as the bile duct brachytherapy system applicator demands a thorough understanding of the CDSCO regulatory framework. This specialized radiotherapy applicator, designed for temporary insertion and computer-controlled placement of radioactive sources in the bile duct, plays a critical role in cancer treatment. Ensuring timely and compliant approval through the Central Drugs Standard Control Organization (CDSCO) is essential for manufacturers and importers aiming to establish a foothold in India’s healthcare sector.
CDSCO Regulatory Framework for Radiotherapy Devices
CDSCO governs the import, manufacture, and sale of medical devices in India under the Medical Device Rules, 2017. Radiotherapy devices, including brachytherapy applicators, fall under specific scrutiny due to their direct impact on patient safety and radiation exposure risks. The bile duct brachytherapy system applicator is notified under File No. 29/Misc./03/2020-DC (180) dated 6.8.2021, incorporating it explicitly within the regulatory ambit.
Risk Classification and License Requirements for Class C Devices
Our extensive experience with over 500 successful CDSCO licenses highlights that this device’s risk classification as Class C demands a centralized approval process. Class C devices, classified as moderate to high risk, require an MD9 manufacturing license issued by the Central Licensing Authority. This involves rigorous testing, documentation, and an audit by CDSCO inspectors to ensure compliance with safety and quality standards.
Manufacturing License Process (MD9) for Bile Duct Brachytherapy Applicator
The MD9 license process for Class C devices typically spans 4 to 5 months and includes the following key stages:
- Test License Application (Form MD13): Initiate by applying for a test license, which takes approximately 1.5 to 2 months. This allows product testing in government-recognized laboratories.
- Product Testing: The device must be tested in CDSCO-approved labs for conformity with applicable standards. You can find the list of testing laboratories here.
- Document Preparation and Submission: Compile comprehensive documentation including Device Master File, Plant Master File, Risk Management File, and more.
- Application for Manufacturing License (Form MD7): Submit the detailed application via the CDSCO MD Online Portal for processing.
- Audit by CDSCO Inspectors: Post-application, an audit evaluates manufacturing facilities and quality systems.
- Queries Resolution: Address any clarifications or additional information requested by the authorities.
- Grant of MD9 License: Upon satisfactory review, the license is issued enabling legal manufacture of the device in India.
Manufacturing License Documents Required for MD9
Ensure all documents are meticulously prepared to avoid delays:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire and Pollution No Objection Certificates (NOCs)
- Device Master File – covering design, specifications, and manufacturing process (detailed guide here)
- Plant Master File – outlining manufacturing facility details (see guide)
- Essential Principles Checklist confirming compliance with safety and performance
- Risk Management File showcasing hazard analysis and mitigation strategies (learn more)
- Test Reports from CDSCO-approved laboratories
- Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documents, preferably ISO 13485:2016 certification
Import License Process (MD15) for Bile Duct Brachytherapy Applicator
For importers, an MD15 import license granted by the Central Licensing Authority is mandatory. The process generally takes 5 to 6 months and involves:
- Preparation of documents including Manufacturing License from country of origin, Free Sale Certificate, CE Certificate, and ISO 13485:2016
- Submission of application on the CDSCO MD Online Portal using Form MD14
- Resolution of any queries raised during the review
- Grant of the MD15 license allowing import and sale in India
Import License Documents Required
Key documents for the import license include:
- Valid Manufacturing License from the country of origin
- Free Sale Certificate affirming product commercialization abroad
- CE Certificate or equivalent for compliance
- ISO 13485:2016 Quality Management System certificate
- Device Master File & Plant Master File
- Wholesale Drug License (if applicable)
- Company constitution and authorization letters
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Document Preparation | 2 – 3 weeks |
| Manufacturing License (MD9) | 2 – 3 months |
| Import License (MD15) | 5 – 6 months total |
Proactive document preparation and timely response to queries can shorten these timelines significantly.
Government Fees and Costs for Class C Device Licensing
- MD9 Manufacturing License Application Fee: ₹50,000 per application
- Product Fee: ₹1,000 per product
- Test License Fee (MD13): Nominal, varies by state
- Import License Fees (MD15): Approximately 1,500 per product
Budgeting accurately for these fees upfront helps prevent unexpected financial bottlenecks.
Common Challenges and Solutions
Challenge: Delays due to incomplete documentation or insufficient risk management data.
Solution: Engage experienced regulatory consultants early to develop and review Device Master Files, ensure risk files meet CDSCO expectations, and align test reports with Indian standards.
Challenge: Audit non-compliance due to inadequate QMS or facility readiness.
Solution: Conduct mock audits and gap analyses before official inspections. Ensure ISO 13485 certification is up to date and quality processes are well documented.
Challenge: Confusion over product classification and applicable fees.
Solution: Verify device classification using the Medical Device Classification guide and consult experts to avoid misfiling.
Expert Consultation and Support
With over 25 years of hands-on experience guiding more than 500 companies through CDSCO licensing, we offer tailored regulatory strategies for complex Class C radiotherapy devices. Our services include:
- End-to-end license application management
- Preparation of Device and Plant Master Files
- Risk management implementation aligned with CDSCO norms
- Coordination with notified bodies and testing laboratories
- Audit preparation and post-audit support
Partnering with us ensures your bile duct brachytherapy system applicator meets all regulatory expectations efficiently.
Getting Started with Your CDSCO License Application
- Verify Device Classification: Confirm your device is Class C as per CDSCO norms.
- Gather Preliminary Documents: Assemble company credentials, technical staff details, and facility proofs.
- Apply for Test License: Submit Form MD13 on the CDSCO MD Online Portal to initiate product testing.
- Select Testing Laboratory: Choose an accredited lab from the official list for your device’s radiotherapy components.
- Develop Comprehensive Documentation: Prepare Device Master File, Risk Management File, Plant Master File, and QMS documentation.
- Submit Manufacturing License Application (Form MD7): After successful testing, apply for the MD9 license through the portal.
- Prepare for Audit: Coordinate with CDSCO inspectors and address any observations promptly.
By following these actionable steps and leveraging professional expertise, your bile duct brachytherapy system applicator will be well-positioned for successful regulatory approval and market entry in India.