CDSCO License for Cardiac Thermodilution Catheter
Medical Device Information
Intended Use
A catheter used in thermodilution for in troduction of the cold liquid indicator i nto thecardiovascular system or for the assessment of a patient’s hemodynamic condition through simultaneous right atrial, right ventricular, and pulmonary artery or wedge pressure monitoring, cardiac output determination, and for infusing solutions.
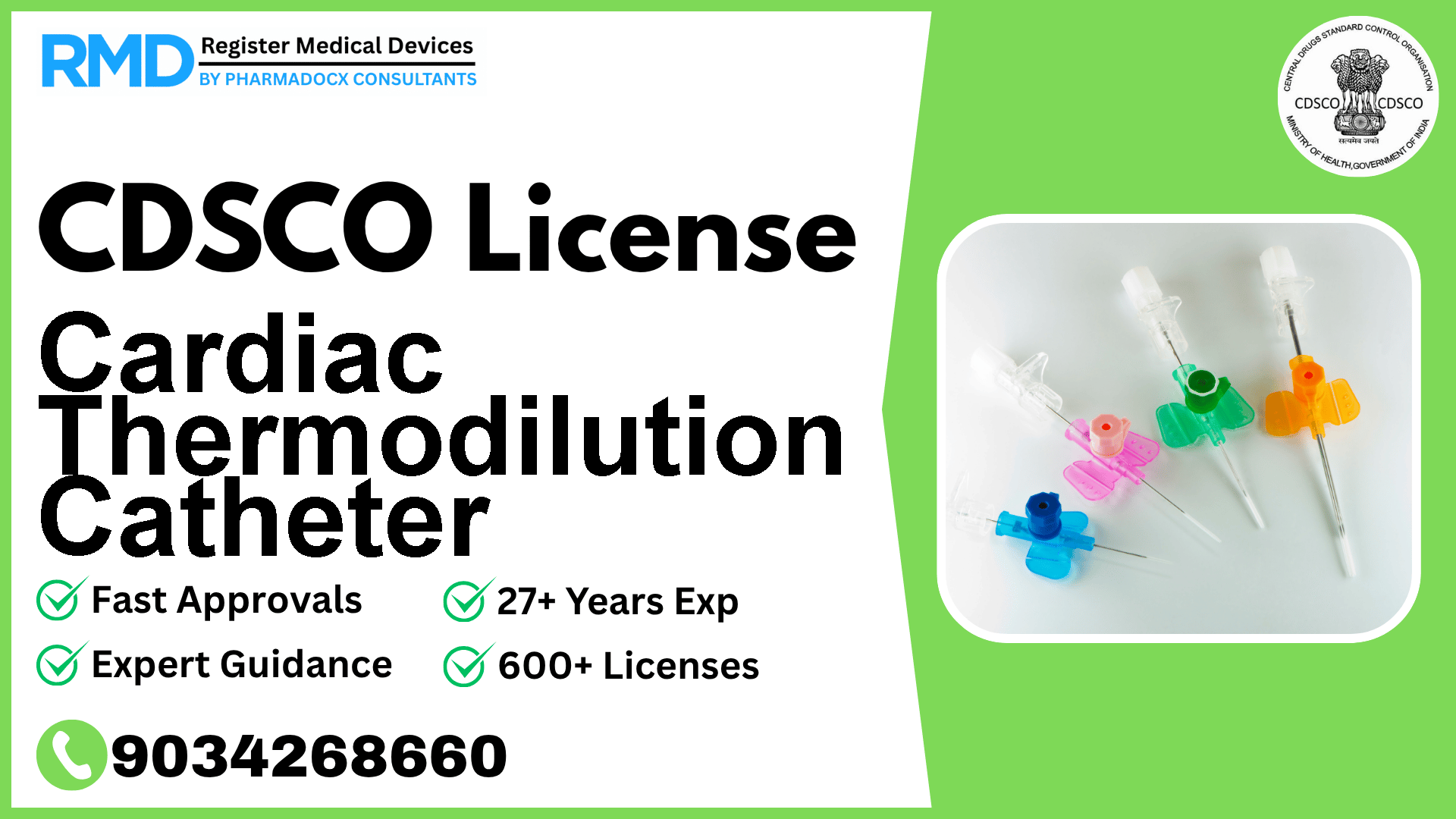
Comprehensive CDSCO Licensing Guide for Cardiac Thermodilution Catheters (Class D)
As seasoned regulatory consultants with over 25 years of experience and having successfully guided 500+ companies through the CDSCO licensing maze, we understand the complexities of introducing high-risk medical devices like the Cardiac Thermodilution Catheter into the Indian market. This catheter, used for advanced hemodynamic monitoring and cardiac output determination, requires stringent regulatory compliance due to its Class D risk classification.
Understanding the CDSCO Regulatory Framework for Cardiac Thermodilution Catheters
The Central Drugs Standard Control Organization (CDSCO) governs the import, manufacture, and sale of medical devices in India under the Medical Device Rules (MDR) 2017. The Cardiac Thermodilution Catheter falls under the Class D category — the highest risk class — because it is invasive and critical for patient monitoring and treatment.
This classification mandates rigorous scrutiny by the Central Licensing Authority and compliance with comprehensive documentation and testing requirements.
Risk Classification and License Requirements for Class D Catheters
- Device Classification: Class D (highest risk)
- Applicable License: MD9 Manufacturing License (Application Form MD7)
- Regulatory Authority: Central Licensing Authority (CDSCO HQ)
- Process Duration: Approximately 4-5 months
For importers, an MD15 Import License is required, also granted by CDSCO Central Authority, with a longer timeline of 5-6 months.
Manufacturing License Process for Cardiac Thermodilution Catheter (MD9 License)
The manufacturing license process for Class D devices involves multiple stages:
- Test License (Form MD13): Obtain a test license to manufacture the catheter for testing purposes. This takes about 1.5-2 months.
- Product Testing: Get the catheter tested at CDSCO-approved government laboratories. You can find the updated list of testing laboratories on the CDSCO portal.
- Document Preparation: Prepare comprehensive documentation, including Device Master File, Plant Master File, Risk Management File, and Quality Management System (QMS) documents.
- License Application (Form MD7): Submit the manufacturing license application through the CDSCO MD Online Portal.
- Audit by CDSCO Inspectors: The CDSCO will conduct a detailed audit of your manufacturing facility and QMS.
- Query Resolution: Address any queries or deficiencies raised by the CDSCO or audit team.
- Grant of License (Form MD9): Upon successful completion, the manufacturing license will be granted.
Manufacturing License Documents Required for Cardiac Thermodilution Catheter
Prepare the following key documents meticulously:
- Company Constitution (e.g., Incorporation Certificate)
- Proof of ownership or lease agreement of manufacturing premises
- Technical staff qualifications and experience
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF): Detailed design and manufacturing process documentation (Device Master File Guide)
- Plant Master File (PMF): Details of manufacturing facilities and quality systems (Plant Master File Guide)
- Essential Principles Compliance Checklist
- Risk Management File demonstrating compliance with ISO 14971 principles (Risk Management)
- Product Test Reports from CDSCO-approved labs
- Product labels and Instructions For Use (IFU)
- Quality Management System documents (ISO 13485:2016 certification recommended)
Import License Process (MD15) for Cardiac Thermodilution Catheters
If you plan to import the catheter into India, follow these steps:
- Document Preparation: Collect all required documents including manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate, DMF, PMF, and wholesale license.
- Application Submission: File Form MD14 for the MD15 import license via the CDSCO MD Online Portal.
- Queries and Review: Respond promptly to any queries raised by CDSCO during document scrutiny.
- License Grant: After satisfactory review, MD15 license is issued.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate from the country of origin
- ISO 13485:2016 and CE Certificates
- Device Master File and Plant Master File
- Wholesale license issued by state authority
- Company Constitution documents
Timeline and Processing Duration
| License Type | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| MD9 Manufacturing License | 4 - 5 months (including test license and audit) |
| MD15 Import License | 5 - 6 months |
Government Fees and Costs
| License Type | Application Fee | Per Product Fee |
|---|---|---|
| MD9 Manufacturing | Rs 50,000 | Rs 1,000 |
| MD15 Import (Class D) | $3,000 per site | $1,500 per product |
Note: Fees are subject to change. Ensure payment is made via the CDSCO portal during application.
Common Challenges and Proven Solutions
Challenge 1: Delays in product testing due to lab backlogs
- Solution: Initiate testing early after test license issuance and engage with multiple CDSCO-approved labs.
Challenge 2: Incomplete documentation causing repeated query cycles
- Solution: Use checklists for all documents and pre-audit internal reviews. Leverage expert consultation for document preparation.
Challenge 3: Audit non-compliance findings
- Solution: Maintain a robust QMS aligned with ISO 13485 and remediate issues promptly.
Expert Consultation and Support
Navigating the CDSCO licensing process for high-risk devices like the Cardiac Thermodilution Catheter requires specialized expertise. Our team offers tailored support:
- End-to-end license application management
- Documentation preparation and gap analysis
- Coordination with notified bodies and labs
- Training on regulatory compliance and QMS implementation
Our proven track record of assisting over 500 companies ensures you meet all regulatory expectations efficiently.
Getting Started with Your CDSCO License Application for Cardiac Thermodilution Catheter
- Assess Your Device Classification: Confirm your device is Class D using the Medical Device Classification tool.
- Register on CDSCO MD Online Portal: Create an account and familiarize yourself with the application process.
- Prepare Test License Application (MD13): Initiate manufacturing for testing.
- Select a CDSCO-approved Testing Laboratory: Early engagement can expedite testing (Testing Laboratories).
- Compile Documentation: Use our Device Master File and Plant Master File guides to organize documentation.
- Schedule Internal Audits: Prepare for CDSCO inspection with internal quality audits.
- Submit Manufacturing License Application (MD7) via portal: After test license completion and testing.
- Maintain Open Communication: Respond promptly to CDSCO queries.
By following these practical steps with expert support, you can successfully secure your MD9 license and manufacture the Cardiac Thermodilution Catheter in India, bringing this critical device to market with confidence and compliance.
For personalized guidance, feel free to contact our regulatory team to streamline your CDSCO licensing journey.