CDSCO License for Colonoscope stiffener
Medical Device Information
Intended Use
A dedicated stiff wire that is inserted into a flexible colonoscope to allow the physician to increase the stiffness of the colonoscope when extra rigidity is required during a colonoscopy.
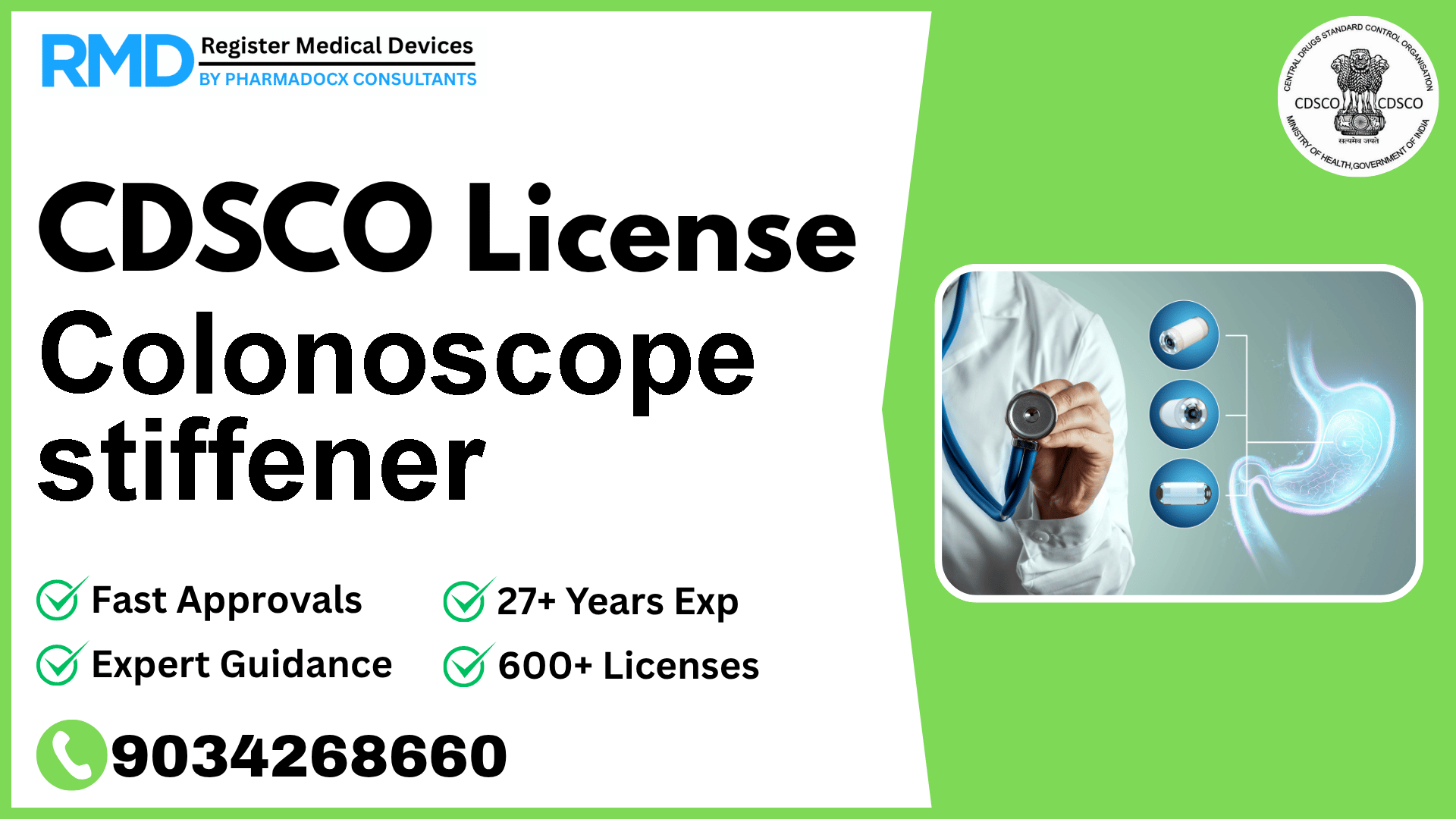
Introduction: Understanding the Colonoscope Stiffener and Its Regulatory Significance
The colonoscope stiffener is a specialized medical device designed to enhance the rigidity of flexible colonoscopes during gastroenterological procedures. By inserting a dedicated stiff wire into the colonoscope, physicians gain better control and maneuverability, which is crucial in complex colonoscopies. Given its clinical importance and patient safety implications, this device is classified as a Class B medical device under the Indian CDSCO framework, mandating compliance with specific regulatory requirements.
Navigating the CDSCO licensing process for a colonoscope stiffener can be complex for manufacturers and importers aiming to enter the Indian market. With over 25 years of experience and having assisted more than 500 companies, we provide actionable insights to streamline this journey.
CDSCO Regulatory Framework for Colonoscope Stiffener
The Central Drugs Standard Control Organization (CDSCO) governs medical device regulation in India. The colonoscope stiffener falls under Class B risk category, as per the notification 29/Misc./03/2020-DC (182) dated 27.09.2021. This classification is based on the device’s intended use and risk factors associated with its clinical application.
Manufacturers of Class B devices must obtain the MD5 manufacturing license, issued by the State Licensing Authority, ensuring compliance with quality and safety standards before commercial distribution.
Risk Classification and License Requirements for Colonoscope Stiffener
Class B devices are considered low moderate risk, necessitating a robust quality management system and thorough documentation. For colonoscope stiffeners, the following apply:
- License Type: MD5 Manufacturing License
- Application Form: MD3
- Issuing Authority: State Drug Control Authority
- Total Processing Time: Approximately 3 to 4 months
- Fees: ₹5,000 per application + ₹500 per product
This classification demands a test license (MD13) prior to full license application, product testing in CDSCO-approved labs, and an audit by a notified body.
Manufacturing License Process (MD5) for Colonoscope Stiffener
The MD5 license process unfolds in a stepwise manner:
Apply for Test License (Form MD13): Initiate by submitting an application for a test license to legally produce and test the colonoscope stiffener. The test license period typically spans 1.5 to 2 months.
Product Testing: Conduct mandatory product testing at CDSCO-approved laboratories to demonstrate compliance with applicable standards. Refer to the list of testing laboratories for approved facilities.
Documentation Preparation: Compile the required technical and quality documents, including Device Master File and Plant Master File.
Submit MD5 License Application (Form MD3): File the manufacturing license application through the CDSCO MD Online Portal.
Audit by Notified Body: Undergo an on-site audit by a notified body to verify compliance with Good Manufacturing Practices (GMP). You can check the list of notified bodies authorized for this purpose.
Address Queries: Promptly respond to any queries or observations raised by the licensing authority or notified body.
Grant of License (Form MD5): Upon satisfactory review, the State Licensing Authority grants the manufacturing license allowing commercial production.
Manufacturing License Documents Required for Colonoscope Stiffener
A comprehensive documentation package is critical for a successful application. Key documents include:
- Company Constitution (Incorporation Certificate, Partnership Deed, etc.)
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire Safety NOC
- Pollution Control NOC
- Device Master File (DMF): Detailed design and manufacturing information. Our Device Master File guide offers practical advice.
- Plant Master File (PMF): Description of the manufacturing facility and processes. Learn more through our Plant Master File guide.
- Essential Principles Compliance Checklist
- Risk Management File demonstrating hazard analysis and mitigation measures. Refer to our Risk Management guide.
- Product Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) Documentation conforming to ISO 13485:2016
Import License Process (MD15) for Colonoscope Stiffener
For importers, obtaining the MD15 license is mandatory before bringing colonoscope stiffeners into India. The process is as follows:
Document Preparation: Assemble all required import documents including manufacturing license from the country of origin, Free Sale Certificate, CE certificate or equivalent, and QMS certifications.
Application Submission: File the application in Form MD14 via the CDSCO MD Online Portal.
Department Review and Queries: The Central Licensing Authority reviews the application and may raise queries.
Grant of Import License (Form MD15): Upon satisfactory review, the import license is issued.
Import License Documents Required for Colonoscope Stiffener
- Valid Manufacturing License from country of origin
- Free Sale Certificate
- ISO 13485:2016 Certification
- CE Certificate or equivalent regulatory approval
- Device Master File
- Plant Master File
- Wholesale Drug License
- Company Constitution
Timeline and Processing Duration
- Test License (MD13): 1.5 to 2 months
- Product Testing: 1 to 1.5 months (can overlap with test license timeline)
- MD5 License Application and Audit: 1.5 to 2 months
Total Estimated Duration: Approximately 3 to 4 months from start to finish
Delays often arise from incomplete documentation or delayed audit scheduling, so early preparation is advised.
Government Fees and Costs
- Test License Fee: Included within application
- MD5 License Fee: ₹5,000 per application + ₹500 per product
- Notified Body Audit Fees: Varies by auditor, typically ₹50,000 to ₹1,00,000
- Product Testing Fees: Depends on testing scope and lab rates, generally ₹20,000 to ₹50,000 per product
Budgeting for these expenses upfront reduces surprises.
Common Challenges and Solutions
Challenge: Incomplete or inconsistent documentation
- Solution: Use detailed checklists and professional document templates for Device Master and Plant Master Files.
Challenge: Scheduling delays for audits
- Solution: Engage notified bodies early and confirm audit dates well in advance.
Challenge: Test lab backlog causing product testing delays
- Solution: Identify multiple approved labs and book testing slots early.
Challenge: Technical staff qualifications not meeting CDSCO standards
- Solution: Hire experienced regulatory professionals and maintain updated training records.
Expert Consultation and Support
With over 25 years supporting more than 500 medical device companies, we understand the nuances of CDSCO licensing. Our experts assist in:
- Preparing and reviewing technical documents
- Coordinating with notified bodies and testing labs
- Managing submission timelines and government interactions
- Resolving queries swiftly to expedite approvals
Partnering with experienced consultants can reduce your approval time and mitigate compliance risks.
Getting Started with Your CDSCO License Application for Colonoscope Stiffener
To initiate your license application smoothly:
- Register your company on the CDSCO MD Online Portal.
- Conduct a gap analysis of your existing quality system and documentation against CDSCO requirements.
- Prepare your Device Master File and Plant Master File with detailed process and design controls.
- Apply promptly for the test license (MD13) to begin manufacturing and testing legally.
- Schedule product testing with CDSCO-approved laboratories early.
- Engage a notified body for audit preparation and scheduling.
- Submit the MD5 license application (Form MD3) once testing and documentation are complete.
- Monitor application status regularly and respond promptly to any queries.
Taking these practical steps will help ensure a seamless pathway to market authorization for your colonoscope stiffener in India.
For detailed assistance and tailored consulting on your Colonoscope stiffener licensing journey, contact us today and leverage our proven expertise.