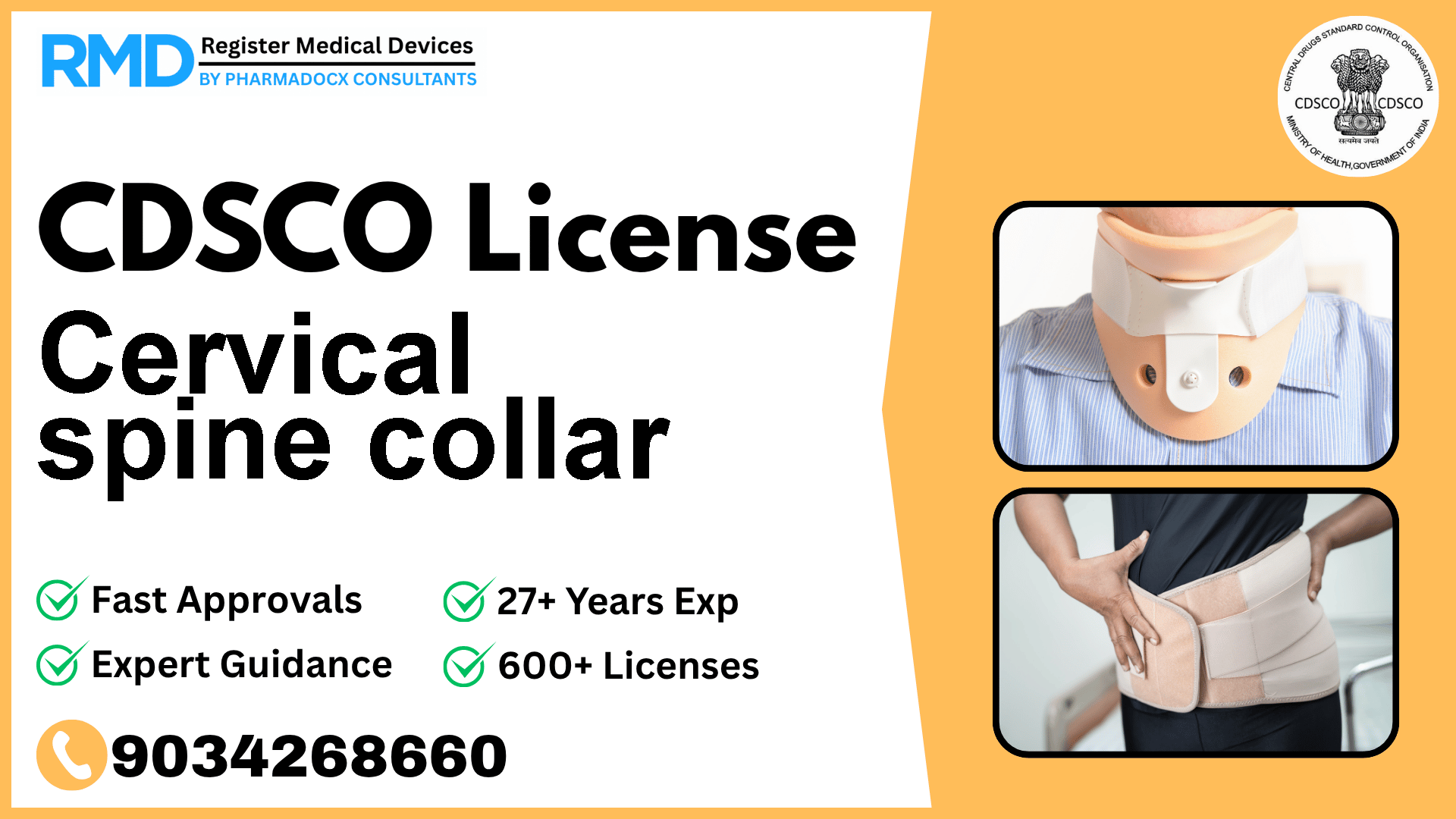
CDSCO License for Cervical spine collar
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
Intended to support or immobilize the cervical spine to treat deformities, fractures, sprains, or strains (often to treat whiplash resulting from an automobile accident).
Comprehensive Guide to CDSCO Licensing for Cervical Spine Collar (Class A Medical Device)
At our consultancy, with over 25 years of experience and 500+ successful CDSCO licensing projects, we understand the complexities manufacturers face when entering the Indian medical device market. The cervical spine collar, a Class A physical support device designed to immobilize the cervical spine for trauma care such as whiplash injuries, requires precise regulatory compliance under the CDSCO framework.
CDSCO Regulatory Framework for Cervical Spine Collars
The Central Drugs Standard Control Organization (CDSCO) regulates medical devices in India under the Medical Devices Rules, 2017. Devices like cervical spine collars are notified under File No. 29/Misc./03/2020-DC (202) dated 26.7.2021, classifying them as Class A – low risk devices.
Risk Classification and License Requirements for Class A Devices
Cervical spine collars fall under Class A, indicating minimal potential risk to patients. This classification mandates obtaining an MD5 manufacturing license issued by the State Licensing Authority. The MD5 license process involves a stepwise approach including a test license, product testing, audit, and final approval.
For importers, an MD15 import license from the Central Licensing Authority is required to legally import these devices.
Stepwise Manufacturing License Process (MD5) for Cervical Spine Collar
Test License Application (Form MD13):
- Duration: 1.5 to 2 months
- Purpose: Permission to produce the device sample for testing
Product Testing:
- Conducted at CDSCO-approved laboratories
- Testing ensures compliance with safety and performance standards
- You can find a list of CDSCO Testing Laboratories here.
Document Preparation:
- Assemble mandatory documents such as Device Master File, Plant Master File, Risk Management File, Essential Principles Checklist, and Quality Management System (QMS) documents.
License Application Submission (Form MD3):
- Submit through the CDSCO MD Online Portal
Audit by Notified Body:
- For Class A devices, audit is conducted by notified bodies listed on the Notified Bodies List for MD5 Audit
Resolution of Queries:
- Address any clarifications or additional information requests from the licensing authority or notified body promptly.
Grant of Manufacturing License (Form MD5):
- Upon satisfactory audit and document review
Essential Documents Required for MD5 License
- Certificate of Incorporation or Company Constitution
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Details and qualifications of Technical Staff
- Fire Safety NOC
- Pollution Control Board NOC
- Device Master File (DMF) – detailing design, materials, and manufacturing process (Guide here)
- Plant Master File (PMF) – outlining facility layout and equipment (Guide here)
- Essential Principles Checklist – compliance with Indian Medical Device Rules
- Risk Management File – hazard analysis and mitigation (Learn more)
- Test Reports from Government-Approved Laboratories
- Labels and Instructions for Use (IFU)
- Quality Management System Documents (e.g., ISO 13485 certificates, SOPs)
Import License Process (MD15) for Cervical Spine Collar
Importers must apply for an MD15 import license through the Central Licensing Authority. Note that for Class A devices, no test license is required prior to application.
Steps:
- Prepare required documents including manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016 certificate, CE Certificate, Device Master File, Plant Master File, and Wholesale License.
- Submit application on the CDSCO MD Online Portal
- Respond to any department queries
- Receive license on Form MD15
Timeline and Processing Duration
MD5 Manufacturing License: Approximately 3 to 4 months total
- Test license: 1.5 to 2 months
- Product testing and document preparation: 1 month
- Audit and query resolution: 1 to 1.5 months
MD15 Import License: Approximately 5 to 6 months
Government Fees and Costs
MD5 License:
- Application Fee: Rs 5,000 per application
- Product Fee: Rs 500 per product
MD15 Import License for Class A:
- Site Fee: $1,000
- Product Fee: $50 per product
These costs cover application processing, audit, and licensing fees.
Common Challenges and Practical Solutions
Delayed Test Reports: Government labs may experience backlogs. We recommend early sample submission and parallel preparation of documents to avoid delays.
Incomplete Documentation: Missing technical staff qualifications or incomplete Device Master Files can stall approval. Use our comprehensive checklists and templates to ensure completeness.
Audit Non-compliance: Many applicants falter during audits due to inadequate QMS or facility issues. Pre-audit consultancy and mock audits can greatly improve outcomes.
Responding to Queries: Timely and detailed response to CDSCO queries prevents license delays. We advise maintaining a dedicated regulatory liaison team.
Expert Consultation and Support
With decades of experience, our team offers end-to-end support—from initial classification and documentation to audit preparation and submission. We have successfully guided over 500 manufacturers and importers through CDSCO licensing for Class A devices like cervical spine collars.
Getting Started with Your CDSCO License Application
- Verify Classification and Intended Use: Confirm your cervical spine collar falls under Class A.
- Initiate Test License (MD13) Application: Apply early via the CDSCO MD Online Portal.
- Engage Approved Testing Labs: Coordinate testing as soon as test license is granted.
- Prepare Comprehensive Documentation: Utilize our Device Master File and Plant Master File guides to compile required documents.
- Schedule Notified Body Audit: Select from the list of notified bodies for your audit.
- Submit MD5 Application (Form MD3): Once testing and documentation are ready.
- Address Queries Promptly: Ensure a dedicated team is ready to respond.
Embarking on the CDSCO licensing journey for your cervical spine collar need not be daunting. With the right expertise and proactive planning, you can achieve market authorization efficiently and compliantly. Contact us today for personalized assistance tailored to your medical device licensing needs.