CDSCO License for Cervicothoracolumbosacral spine orthosis
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
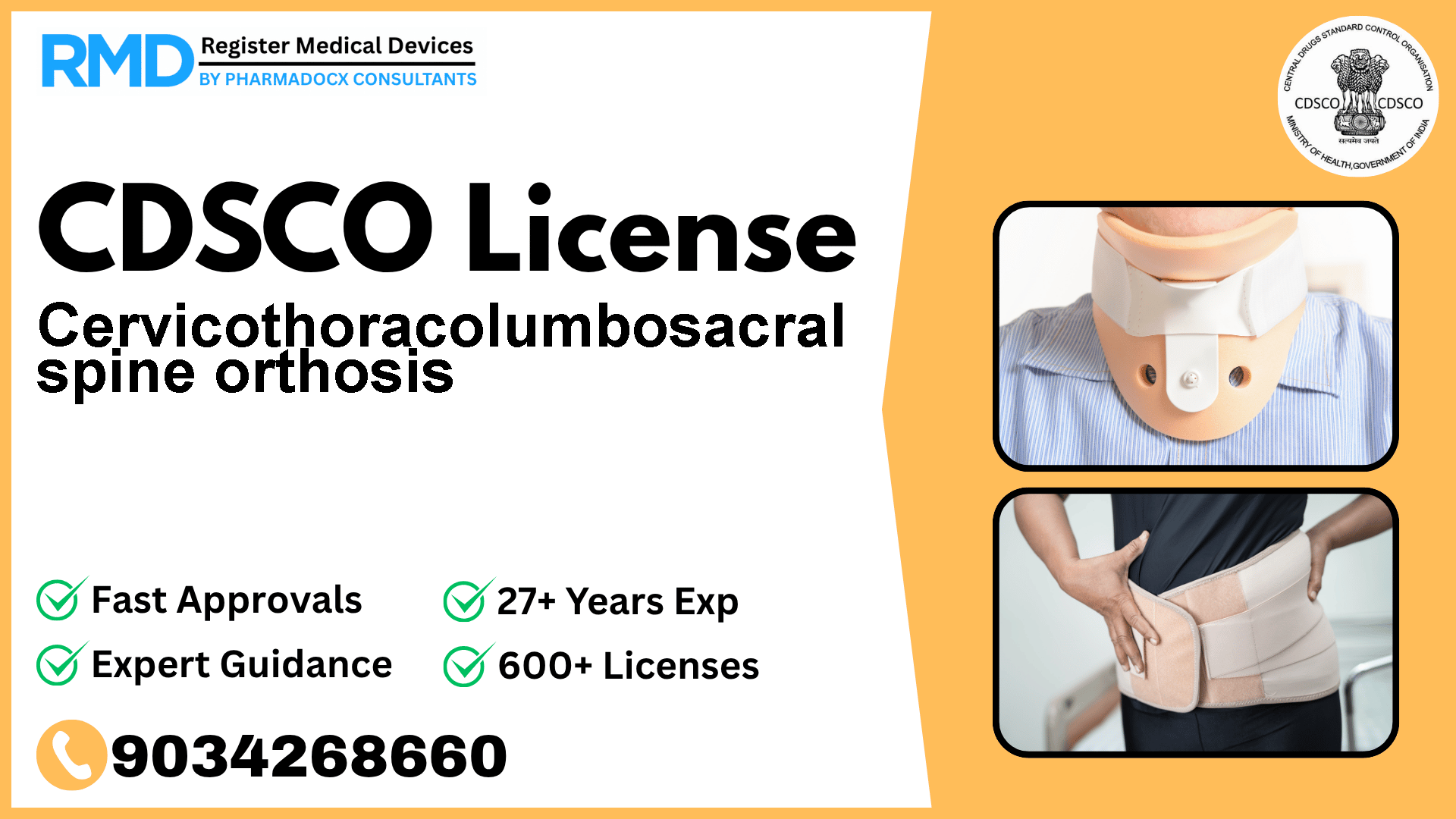
Intended to encompass the cervicothoracolumbosacral spine region of the neck and trunk.
Comprehensive Guide to CDSCO Licensing for Cervicothoracolumbosacral Spine Orthosis
Navigating the regulatory landscape for medical devices in India can be complex, especially when it comes to obtaining the correct CDSCO license. With over 25 years of experience and having successfully assisted 500+ companies, we bring you a practical, detailed guide tailored specifically for the Cervicothoracolumbosacral spine orthosis, a Class A physical support device.
Understanding the Cervicothoracolumbosacral Spine Orthosis and Its Regulatory Importance
This orthosis is designed to provide physical support to the cervicothoracolumbosacral region, encompassing the neck and trunk. Classified under Class A (low risk) devices by CDSCO, it falls under the physical support category as per the notification File No. 29/Misc./03/2020-DC (202), dated 26.7.2021.
Obtaining a CDSCO manufacturing license is mandatory before you can legally manufacture or import this device in India, ensuring compliance with quality and safety standards that protect patients and healthcare providers.
CDSCO Regulatory Framework for Cervicothoracolumbosacral Spine Orthosis
The Central Drugs Standard Control Organisation (CDSCO) regulates medical devices under the Medical Device Rules 2017. For Class A devices like the cervicothoracolumbosacral spine orthosis, the State Licensing Authority grants the manufacturing license (MD5). The entire process involves multiple steps including testing, documentation, and audit.
Risk Classification and License Requirements
- Risk Class: A (Low risk)
- Applicable License: MD5 (Manufacturing license for Class A & B devices)
- Governing Authority: State Licensing Authority
For more details on medical device classification, you can refer to our Medical Device Classification guide.
Manufacturing License Process (MD5) for Class A Devices
Test License (MD13): Before applying for the manufacturing license, you must obtain a test license to conduct product testing. This process takes approximately 1.5 to 2 months.
Product Testing: Testing must be conducted at CDSCO-approved laboratories. This ensures your orthosis meets safety and performance standards. Check the list of approved Testing Laboratories.
Document Preparation: Compilation of all required documents including Device Master File, Plant Master File, risk management files, and QMS documentation.
Application Submission: File your application using Form MD3 on the CDSCO MD Online Portal.
Audit by Notified Body: A notified body accredited by CDSCO will audit your manufacturing facility and quality systems. The list of Notified Bodies is publicly available.
Query Resolution: Address any queries raised by the licensing authority or the notified body.
Grant of License: Upon successful audit and query resolution, the license is granted on Form MD5.
Manufacturing License Documents Required for Cervicothoracolumbosacral Spine Orthosis
- Company Constitution and Incorporation Documents
- Proof of Ownership or Rent Agreement of Manufacturing Premises
- Technical Staff Qualification and Experience Details
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF): Detailed design and manufacturing process documents. Our Device Master File guide can assist you.
- Plant Master File (PMF): Details of your manufacturing facility and quality systems. Refer to our Plant Master File guide.
- Essential Principles Checklist
- Risk Management File specific to the orthosis device. Learn more about Risk Management.
- Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documentation (e.g., ISO 13485 compliance)
Import License Process (MD15) for Cervicothoracolumbosacral Spine Orthosis
For importers, the process is handled by the Central Licensing Authority. Although Class A devices generally have a simpler import licensing process, the MD15 license requires:
- Manufacturing license of the product from the country of origin
- Free Sale Certificate
- ISO 13485:2016 certificate
- CE Certificate or equivalent
- Device and Plant Master Files
- Wholesale license for distribution
- Company Constitution
The application is filed using Form MD14 on the CDSCO MD Online Portal. Expect a timeline of 5-6 months for the entire import license process.
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 1 - 1.5 months |
| Document Preparation | 1 month |
| License Application (MD3) | Immediate upon document readiness |
| Audit by Notified Body | 1 month |
| Query Resolution | 1 month |
| Total Time for MD5 | 3 - 4 months |
Government Fees and Costs
- Application fee: Rs. 5,000 per application
- Product fee: Rs. 500 per product
- Additional costs include:
- Testing fees charged by government-approved labs
- Audit fees payable to the notified body
- Costs for document preparation and consultancy if outsourced
Common Challenges and Solutions
- Incomplete Documentation: Ensure all files like DMF, PMF, and risk management files are complete and compliant. Using structured templates can help.
- Delays in Testing: Schedule testing early and choose CDSCO-approved labs with shorter lead times.
- Audit Non-compliance: Pre-audit internal checks and gap analysis can prepare your facility to meet notified body standards.
- Query Management: Respond thoroughly and promptly to queries to avoid delays.
Expert Consultation and Support
Our extensive experience with over 500 successful CDSCO licensing projects enables us to provide:
- Tailored document preparation support
- Liaison with notified bodies and CDSCO officials
- Pre-audit readiness assessments
- End-to-end project management from test license to final approval
Getting Started with Your CDSCO License Application
- Assess your product classification to confirm Class A status.
- Initiate the test license (Form MD13) application via the CDSCO MD Online Portal.
- Engage CDSCO-approved testing laboratories early to book testing slots.
- Prepare your Device Master File and Plant Master File in alignment with regulatory expectations.
- Plan for the notified body audit by selecting from the list of notified bodies.
- Compile all technical and legal documentation as per the checklist outlined above.
- Submit your manufacturing license application (Form MD3) through the online portal.
- Maintain proactive communication with regulators and auditors to expedite query resolution.
By following these precise steps and leveraging expert guidance, manufacturers of cervicothoracolumbosacral spine orthosis can efficiently secure their CDSCO MD5 license, ensuring timely market entry and regulatory compliance.
For detailed assistance, consult our comprehensive MD5 License Guide and connect with our regulatory experts to streamline your licensing journey.