CDSCO License for Collar and cuff arm sling material
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
Fabric and form composite material intended to immobilize forearm, elbow, humerus or shoulder injuries.
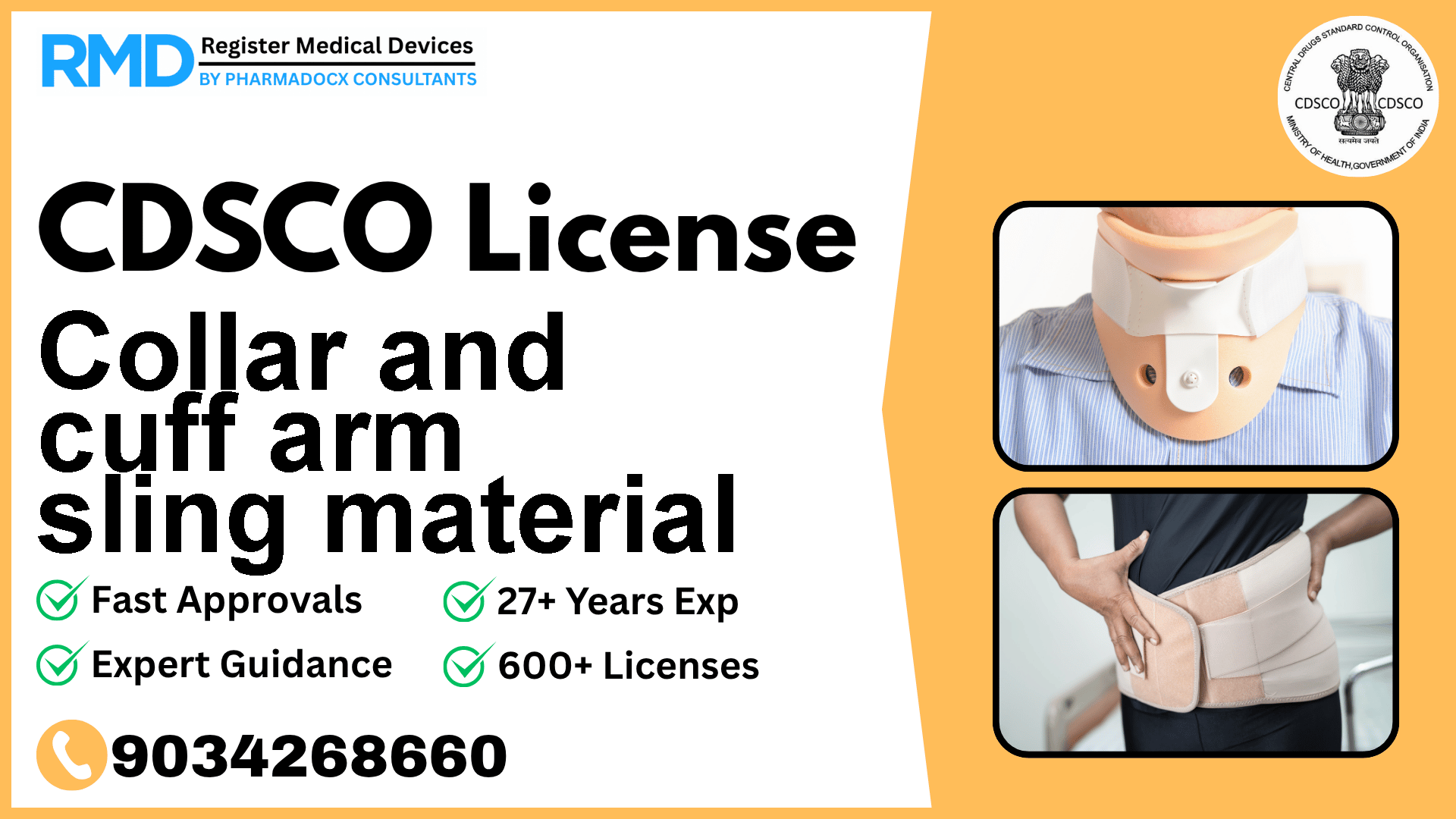
Comprehensive Guide to CDSCO Licensing for Collar and Cuff Arm Sling Material (Class A Medical Device)
As a trusted regulatory consultancy with over 25 years of experience and having successfully supported 500+ manufacturers and importers, we understand the critical importance of securing the right CDSCO license for your medical device. This guide is tailored specifically for the Collar and Cuff Arm Sling Material, a Class A physical support device used to immobilize forearm, elbow, humerus, or shoulder injuries.
Introduction: Device Overview and Regulatory Importance
The Collar and Cuff Arm Sling is a fabric and form composite material designed to provide immobilization for various upper limb injuries. Classified as a Class A medical device under the Indian regulatory framework, it falls under low-risk physical support devices. Despite its classification as low risk, obtaining the correct CDSCO license is mandatory before manufacturing or marketing this device in India.
Compliance with CDSCO regulations ensures patient safety, market credibility, and legal distribution. The device is covered under the notification No. 29/Misc./03/2020-DC (202) dated 26.7.2021, which highlights its regulatory applicability.
CDSCO Regulatory Framework for Collar and Cuff Arm Sling
The Central Drugs Standard Control Organization (CDSCO) governs medical devices through a structured licensing framework. For Class A devices like the Collar and Cuff Arm Sling, the licensing falls under the State Licensing Authority with the MD5 Manufacturing License process.
The regulatory steps include:
- Obtaining a Test License (Form MD13)
- Product Testing from CDSCO-approved laboratories
- Submission of the Manufacturing License Application (Form MD3)
- Audit by a notified body
- Resolution of queries
- Grant of the MD5 License
Manufacturers must comply with the Essential Principles of Safety and Performance, maintain a Quality Management System (QMS) aligned with ISO 13485, and maintain documentation such as Device Master File and Plant Master File.
Risk Classification and License Requirements
According to CDSCO guidelines, Collar and Cuff Arm Sling Material is classified as Class A – Low Risk. This classification mandates the following licensing:
| Risk Class | License Type | License Authority | Application Form | Estimated Timeline | Fees |
|---|---|---|---|---|---|
| Class A | MD5 License | State Licensing Authority | MD3 | 3-4 months | Rs 5,000 + Rs 500 per product |
Understanding this classification is vital since it dictates the application process, documentation, and compliance requirements. For a detailed understanding of medical device classification, manufacturers can refer to our Medical Device Classification guide.
Manufacturing License Process (MD5 License): Step-by-Step
- Application for Test License (Form MD13):
- Duration: Approximately 1.5 to 2 months
- Purpose: Allows the manufacturer to legally produce the device for testing purposes.
- Product Testing:
- Conducted at CDSCO-approved testing laboratories. You can check the list of Testing Laboratories here.
- Testing validates conformity with applicable standards and safety requirements.
- Preparation of Documentation:
- Compile Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, QMS documents, and other required documents.
- Submission of Manufacturing License Application (Form MD3):
- Submit via the CDSCO MD Online Portal.
- Audit by Notified Body:
- Audit conducted by notified bodies approved by CDSCO. Check the list of notified bodies here.
- Queries and Clarifications:
- Address any queries raised by the licensing authority or notified body promptly.
- Grant of MD5 License (Form MD5):
- Upon satisfactory compliance, the license is granted.
We recommend initiating the process well in advance to accommodate any unforeseen delays during testing or audits.
Manufacturing License Documents Required for Collar and Cuff Arm Sling
For a smooth application process, ensure the following documents are prepared meticulously:
- Company Constitution Documents: Including Certificate of Incorporation, Memorandum and Articles of Association.
- Proof of Ownership or Lease of Manufacturing Premises
- Technical Staff Qualification and Experience Proofs: To demonstrate competent personnel.
- No Objection Certificates: Fire safety and Pollution control NOCs.
- Device Master File (DMF): Detailed device description, design, and specifications. Our Device Master File guide provides comprehensive insights.
- Plant Master File (PMF): Details of manufacturing facilities and processes. Learn more from our Plant Master File guide.
- Essential Principles Checklist: Compliance with safety and performance standards.
- Risk Management File: Risk analysis and mitigation strategies. Refer to our Risk Management guide for best practices.
- Test Reports: From CDSCO-approved labs.
- Labels and Instructions for Use (IFU): In compliance with regulatory requirements.
- Quality Management System Documents: ISO 13485 certification and related procedures.
Import License Process (MD15 License) for Collar and Cuff Arm Sling
For importers of the Collar and Cuff Arm Sling, the MD15 import license is mandatory. This license is issued by the Central Licensing Authority and typically takes 5-6 months.
Key steps include:
- Preparation of import-related documentation such as Manufacturing License from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device Master and Plant Master files.
- Application submission via the CDSCO MD Online Portal.
- Query resolution and final approval.
Detailed guidance is available in our Import License Guide.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate (if applicable)
- Device Master File
- Plant Master File
- Wholesale Drug License (if applicable)
- Company Constitution Documents
Timeline and Processing Duration
| License Type | Process Step | Approximate Duration |
|---|---|---|
| MD5 Manufacturing | Test License (MD13) | 1.5 to 2 months |
| License Application | Product Testing | 1 to 1.5 months |
| Documentation & Audit | 1 to 1.5 months | |
| Query Resolution | 0.5 month | |
| Total Time | 3 to 4 months |
It is advisable to plan for potential delays in testing or audit scheduling. Early engagement with notified bodies can help mitigate timing risks.
Government Fees and Costs
| License Type | Application Fee | Product Fee | Total (Approximate) |
|---|---|---|---|
| MD5 License | Rs 5,000 | Rs 500 per product | Rs 5,500 for one product |
Additional costs include:
- Testing fees charged by laboratories
- Notified body audit fees
- Consultancy fees (if applicable)
By budgeting for these upfront, manufacturers can avoid financial surprises.
Common Challenges and Practical Solutions
- Incomplete Documentation: Many applicants face delays due to missing or inconsistent documents. We recommend using checklists and professional document review services.
- Delays in Product Testing: Testing laboratories can be backlogged; booking slots early is essential.
- Audit Non-compliance: Not following GMP or QMS standards may lead to failed audits. Pre-audit readiness assessments help prevent this.
- Query Resolution Delays: Prompt and clear responses to CDSCO queries can speed up approval.
Our hands-on experience allows us to navigate these challenges efficiently, ensuring smooth licensing.
Expert Consultation and Support
Our team has successfully assisted over 500 companies in obtaining CDSCO licenses for Class A medical devices like the Collar and Cuff Arm Sling. We provide:
- End-to-end application management
- Documentation preparation and review
- Coordination with notified bodies and testing labs
- Audit preparation and training
- Post-license compliance support
Engage with us early in your project to leverage our expertise and avoid costly pitfalls.
Getting Started with Your CDSCO License Application
- Assess your device classification and licensing needs. Confirm your device falls under Class A and requires an MD5 license.
- Prepare your technical and quality documentation. Utilize our detailed guides on Device and Plant Master Files.
- Apply for the Test License (Form MD13) on the CDSCO MD Online Portal.
- Schedule product testing at CDSCO-approved labs. Early booking is critical.
- Engage a notified body for the required audit. Refer to the list of notified bodies.
- Submit your Manufacturing License Application (Form MD3).
- Respond promptly to any queries from CDSCO or auditors.
By following these steps and leveraging expert support, manufacturers can confidently enter the Indian market with their Collar and Cuff Arm Sling materials.
For personalized assistance, reach out to our regulatory experts and ensure your CDSCO license journey is efficient and successful.