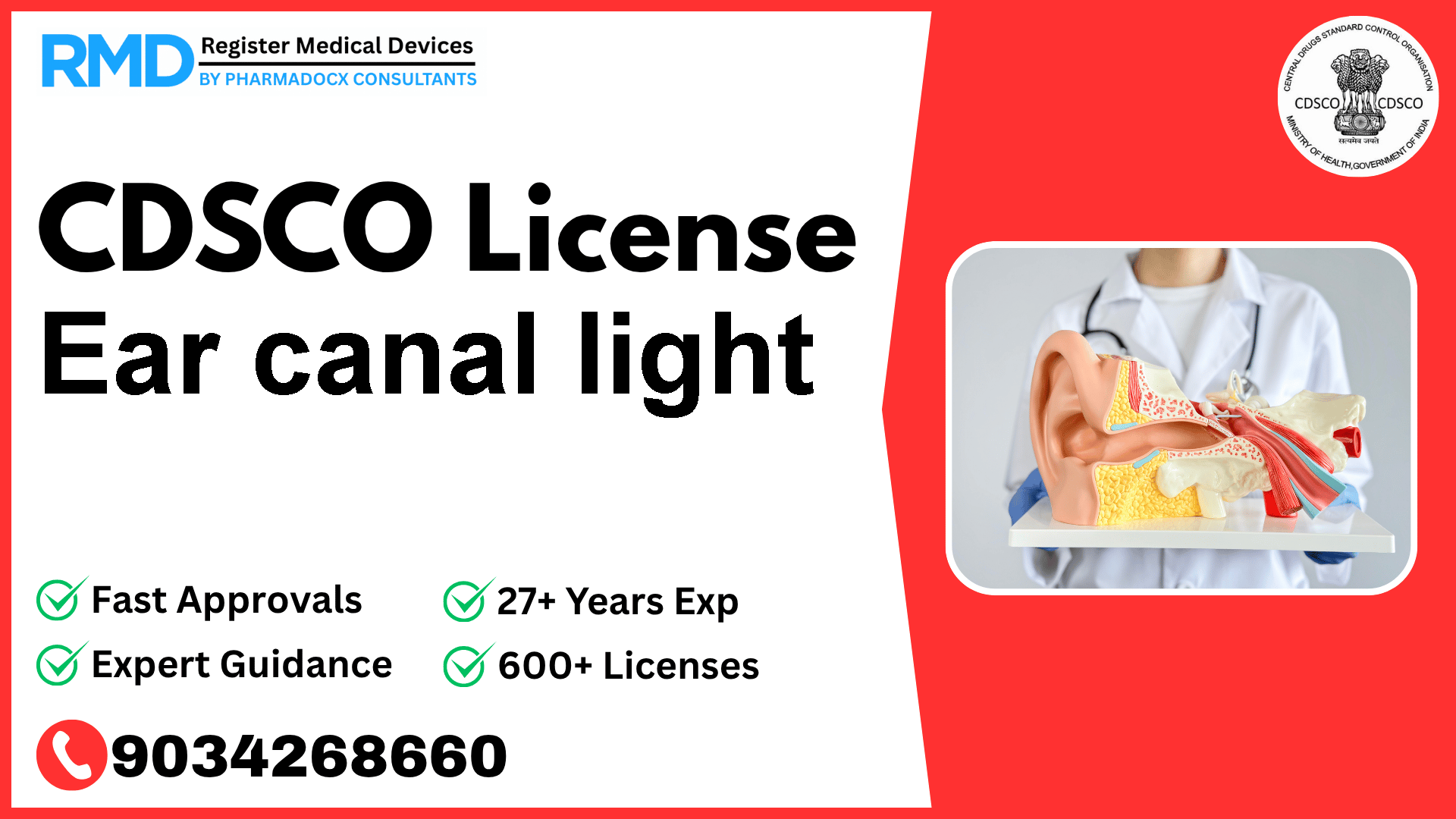
CDSCO License for Ear canal light
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
Intended to illuminate the ear canal.
Comprehensive Guide to CDSCO Licensing for Ear Canal Light (Class A Medical Device)
As a trusted regulatory consultancy with over 25 years of experience assisting 500+ manufacturers and importers, we understand the nuances involved in obtaining CDSCO licenses for medical devices like the Ear Canal Light. This device, classified as Class A under the CDSCO framework, plays a crucial role in ENT diagnostics by illuminating the ear canal effectively and safely. Compliance with CDSCO regulations is not just a legal necessity but a gateway to establishing your footprint in the rapidly expanding Indian medical device market.
CDSCO Regulatory Framework for Ear Canal Light
The Central Drugs Standard Control Organization (CDSCO) regulates all medical devices in India under the Medical Device Rules (MDR) 2017. The Ear Canal Light, categorized under ENT devices and classified as Class A — the lowest risk category — requires a manufacturing license under Form MD5, issued by the State Licensing Authority.
Regulatory compliance ensures product safety, quality, and efficacy, essential for patient safety and market acceptance. The relevant notification for this device is 29/Misc/03/2020-DC(196), dated 06.08.2021.
Risk Classification and License Requirements for Ear Canal Light
Class A devices are considered low risk, demanding adherence to basic regulatory controls. Under the MDR, manufacturing requires an MD5 license (Form MD3 application) approved by the state authority. The process incorporates a mandatory test license (Form MD13), product testing by government-approved laboratories, and an audit by a notified body.
For detailed classification guidelines, manufacturers can refer to the Medical Device Classification resource.
Manufacturing License Process (MD5) for Ear Canal Light
- Apply for Test License (Form MD13): Obtain a test license allowing you to produce the device sample for testing. This step generally takes 1.5 to 2 months.
- Product Testing: Send samples to CDSCO-approved testing laboratories. Testing typically lasts 30-45 days depending on lab capacity and test complexity. Refer to the Testing Laboratories List for accredited labs.
- Document Preparation: Compile essential documents including Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, test reports, labels, IFU, and QMS documents.
- Submit License Application (Form MD3): Apply through the CDSCO MD Online Portal. Include all requisite documents and test reports.
- Audit by Notified Body: An audit by a notified body (see Notified Bodies List) is conducted to verify compliance.
- Respond to Queries: Address any queries from the CDSCO or the notified body promptly.
- Grant of Manufacturing License (Form MD5): Upon satisfactory review and audit, the MD5 license is granted, authorizing manufacturing.
Manufacturing License Documents Required for Ear Canal Light
- Company Constitution and Incorporation Documents
- Proof of Ownership or Lease of Manufacturing Premises
- Qualification and Experience Certificates of Technical Staff
- Fire and Pollution No Objection Certificates (NOCs)
- Device Master File (DMF) detailing design, specifications, and manufacturing process (Device Master File Guide)
- Plant Master File (PMF) describing facility layout and quality systems (Plant Master File Guide)
- Essential Principles Checklist demonstrating compliance with safety and performance standards
- Risk Management File illustrating hazard analysis and risk mitigation (Risk Management)
- Product Test Reports from government-approved laboratories
- Labels and Instructions for Use (IFU)
- Quality Management System Documentation (typically ISO 13485 compliant)
Import License Process (MD15) for Ear Canal Light
While manufacturing licenses are granted by State Authorities, import licenses (MD15) are issued by the Central Licensing Authority. Importers of Ear Canal Light devices must:
- Ensure the device complies with Indian standards
- Submit application via Form MD14 on the CDSCO MD Online Portal
- Provide supporting documents including Free Sale Certificate, ISO 13485 certificate, CE Certificate if applicable, Device and Plant Master Files, and Wholesale License
The import license process takes approximately 5–6 months.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate issued by the regulatory authority of the manufacturing country
- ISO 13485:2016 certification
- CE Certificate (if available)
- Device Master File and Plant Master File
- Wholesale License for distribution
- Company Constitution and Incorporation Documents
Timeline and Processing Duration
| Step | Estimated Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Document Preparation | 2 – 3 weeks |
| License Application Review | 1 month |
| Audit and Query Resolution | 4 – 6 weeks |
| Total Time for MD5 License | 3 – 4 months |
Government Fees and Costs
- Test License (MD13): Approximately Rs. 3000 - Rs. 5000
- MD5 Manufacturing License Application: Rs. 5000 per application
- Per Product Fee: Rs. 500 for each product variant (Ear Canal Light variants if any)
- Testing Fees: Vary based on laboratory and number of tests
- Audit Fees: Payable to notified body, typically between Rs. 25,000 - Rs. 50,000 depending on scope
Common Challenges and Solutions
Challenge: Delays in test report generation due to high demand at government labs.
Solution: Engage early with the labs listed on the Testing Laboratories portal and consider parallel processes such as document preparation.
Challenge: Incomplete or inconsistent documentation leading to queries and rejections.
Solution: Adopt a meticulous document management system; using guides like our Device Master File Guide ensures completeness.
Challenge: Audit non-compliance due to gaps in QMS or plant facilities.
Solution: Pre-audit internal checks aligned with notified body requirements and standards.
Expert Consultation and Support
With our extensive expertise, we provide end-to-end support for:
- Gap analysis and readiness assessment
- Preparation of Device and Plant Master Files
- Assistance with test license applications and coordination with testing labs
- Support during audits and query resolution
- Strategic guidance on regulatory compliance and post-approval vigilance
Our proven track record with 500+ companies means you can navigate CDSCO licensing confidently and efficiently.
Getting Started with Your CDSCO License Application for Ear Canal Light
- Register on the CDSCO MD Online Portal: Create your account at the official portal.
- Initiate Test License Application: Prepare and submit Form MD13 with all preliminary documents.
- Engage Accredited Testing Labs: Schedule product testing as soon as the test license is granted.
- Compile Comprehensive Documentation: Utilize our detailed guides to prepare your Device Master File, Plant Master File, and Risk Management File.
- Schedule Audit with Notified Body: Coordinate the audit early to avoid delays.
- Submit MD5 License Application (Form MD3): Once testing and documentation are complete, submit your application through the portal.
By following these practical steps and leveraging expert support, manufacturers and importers of Ear Canal Light devices can streamline the licensing process, minimize compliance risks, and successfully enter the Indian market. For personalized assistance and detailed project planning, feel free to contact our consultancy team.
For additional insights and stepwise assistance, explore our resources on MD5 License Guide.