CDSCO License for Exophthalmometer
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
An ophthalmic instrument used to measure the degree of exophthalmos.
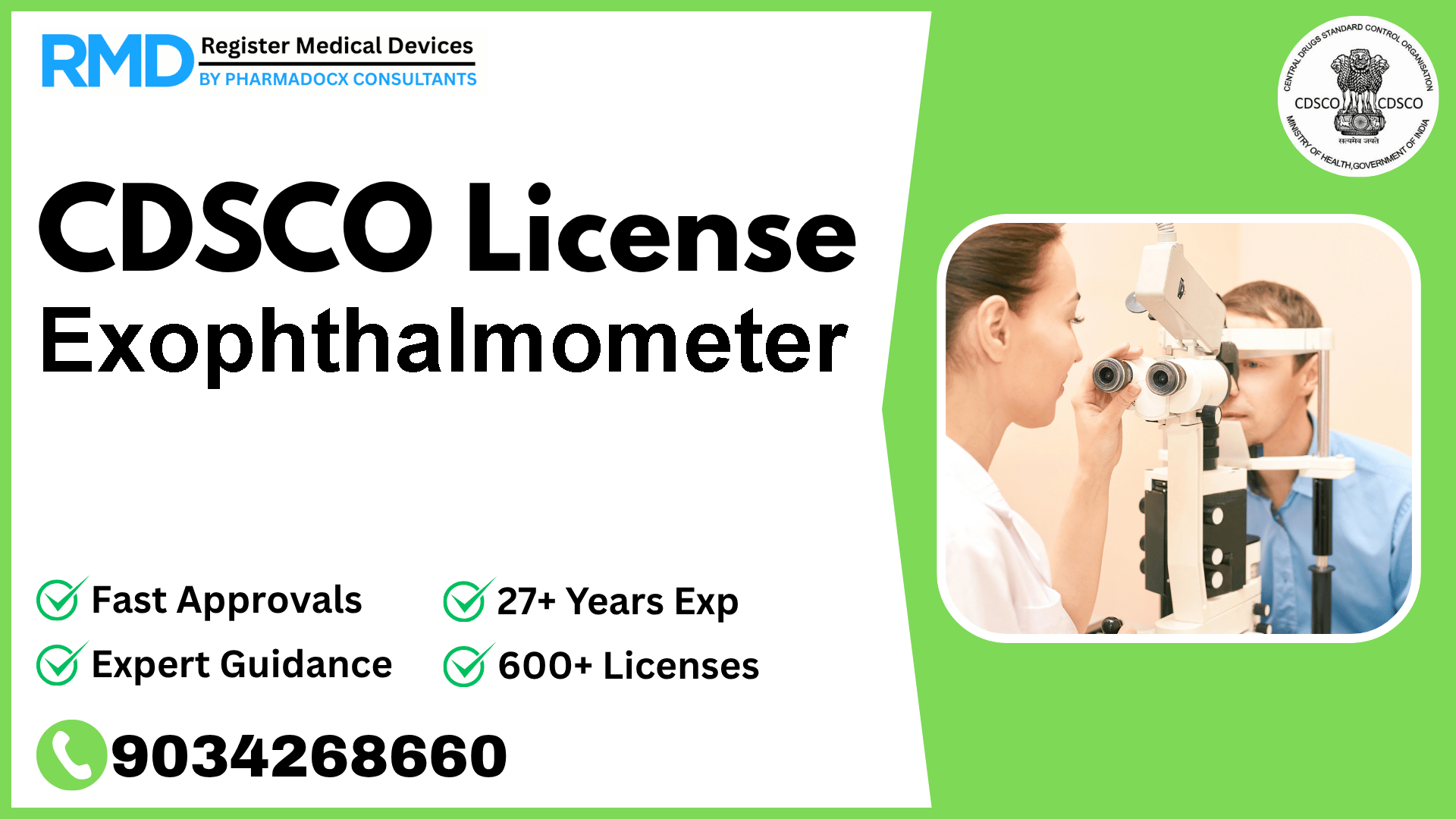
Comprehensive Guide to CDSCO Licensing for Exophthalmometer (Class A Medical Device)
As a trusted regulatory consulting firm with over 25 years of experience and having successfully supported 500+ companies in navigating the Indian medical device regulatory landscape, we understand the nuances involved in obtaining CDSCO licenses. This guide focuses specifically on the Exophthalmometer, a Class A ophthalmic device used to measure the degree of exophthalmos, notified under Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021.
Understanding the Exophthalmometer and Its Regulatory Importance
An Exophthalmometer is a precise ophthalmic instrument essential for diagnosing and monitoring conditions involving eye protrusion. Being a Class A device, it is considered low risk but still requires formal approval to ensure patient safety and compliance with Indian regulations. Obtaining the correct license is critical for legal manufacturing, marketing, and distribution within India.
CDSCO Regulatory Framework for Ophthalmic Devices like Exophthalmometer
In India, the Central Drugs Standard Control Organization (CDSCO) governs medical device approvals under the Medical Device Rules 2017. Devices like the Exophthalmometer fall under the Class A category, which is regulated primarily at the State Licensing Authority level. The licensing process includes a test license, product testing, document submission, and an audit by a notified body.
For more details on device classification, refer to our Medical Device Classification guide.
Risk Classification and License Requirements for Exophthalmometer
- Risk Class: A (Low Risk)
- License Type: MD5 Manufacturing License
- Application Form: MD3
- Regulatory Authority: State Licensing Authority
- Notification Reference: Fts No. 29/MiscJO3/2020-DC (187)
- Intended Use: Measurement of exophthalmos in ophthalmology
Manufacturing License Process for Exophthalmometer (MD5 License)
The licensing journey for Class A devices like the Exophthalmometer involves several key steps:
Test License Application (Form MD13):
- Obtain a test license to manufacture and test prototype batches. This stage takes approximately 1.5 to 2 months.
Product Testing:
- Conduct product testing through government-approved laboratories. Use the list of testing laboratories for accredited centers.
Document Preparation:
- Assemble all technical, quality, and compliance documentation.
License Application (Form MD3):
- Submit the manufacturing license application on the CDSCO MD Online Portal.
Audit by Notified Body:
- An audit of your manufacturing facility is conducted by a notified body. Check the notified bodies list for authorized auditors.
Query Resolution:
- Respond promptly to any queries from CDSCO or the notified body.
Grant of License (Form MD5):
- Upon successful audit and document review, the MD5 license is granted.
Manufacturing License Documents Required for Exophthalmometer
Preparing a comprehensive documentation package ensures a smooth approval process. Key documents include:
- Certificate of Incorporation and Company Constitution
- Proof of ownership or lease of manufacturing premises
- Technical staff qualifications and experience certificates
- Fire and Pollution NOCs from respective authorities
- Device Master File (DMF): Detailed design, specifications, and manufacturing process documentation. Our Device Master File guide provides a step-by-step approach.
- Plant Master File (PMF): Description of the manufacturing facility, equipment, and quality controls. Refer to our Plant Master File guide for assistance.
- Essential Principles Checklist ensuring compliance with Indian regulatory standards
- Risk Management File demonstrating hazard identification and mitigation strategies. Learn more from our Risk Management guide.
- Product Test Reports from government-approved labs
- Labels and Instructions for Use (IFU) compliant with CDSCO guidelines
- Quality Management System (QMS) documentation, preferably ISO 13485:2016 certified
Import License Process for Exophthalmometer (MD15 License)
Although this guide focuses on manufacturing, importers of Exophthalmometers must apply for an MD15 Import License through the Central Licensing Authority.
Key highlights:
- No test license required
- Application via Form MD14 on the CDSCO MD Online Portal
- Typical processing time of 5-6 months
- Requires documentation such as:
- Manufacturing License from country of origin
- Free Sale Certificate
- ISO 13485:2016 certification
- CE Certificate
- Device and Plant Master Files
- Wholesale License
- Company Constitution
For detailed guidance, see our Import License consultant guide.
Timeline and Processing Duration for MD5 License
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 2 - 4 weeks |
| Document Preparation | 3 - 4 weeks |
| License Application | Immediate upon docs readiness |
| Audit by Notified Body | 4 - 6 weeks |
| Query Resolution | 2 - 3 weeks |
| Total Estimated Time | 3 to 4 months |
Government Fees and Costs for Exophthalmometer Manufacturing License
- Application Fee: Rs 5,000 per application
- Product Fee: Rs 500 per product (for Exophthalmometer, one product fee applies)
- Audit and Testing Costs: Variable, approximately Rs 50,000-70,000 depending on notified body and test lab charges
Common Challenges and Practical Solutions
1. Delays in Document Preparation:
- Prepare documents in parallel during test license phase.
- Use templates and checklists to avoid omissions.
2. Testing Lab Appointments:
- Book testing slots early with government-approved labs.
- Clarify test parameters upfront.
3. Audit Non-compliance:
- Conduct internal mock audits before notified body visits.
- Maintain up-to-date QMS and training records.
4. Query Bottlenecks:
- Assign dedicated personnel for quick response.
- Engage regulatory consultants for technical clarifications.
Expert Consultation and Support
With extensive experience in CDSCO regulations, we offer end-to-end support:
- Stepwise application management
- Document drafting and review
- Coordination with test labs and notified bodies
- Training your team on compliance
Our proven track record reduces approval time and minimizes rejections.
Getting Started with Your CDSCO License Application for Exophthalmometer
- Assess your readiness: Ensure your manufacturing setup complies with GMP and ISO 13485:2016.
- Gather core documents: Begin assembling corporate, technical, and quality files.
- Apply for test license (MD13): Submit through the CDSCO MD Online Portal.
- Plan product testing: Identify and liaise with an approved testing laboratory.
- Prepare for audit: Review notified bodies and schedule your audit in advance.
- Submit MD5 application: Complete Form MD3 after successful testing and audit.
Navigating the CDSCO licensing process for the Exophthalmometer can seem daunting, but with structured planning and expert guidance, manufacturers can smoothly enter the Indian market while ensuring compliance and patient safety.
For personalized assistance, contact us to leverage our 25+ years of regulatory expertise and successfully obtain your CDSCO license.
Explore more resources and regulatory updates on the official CDSCO MD Online Portal.