CDSCO License for Fundus-imaging ophthalmic diode laser system
Medical Device Information
Intended Use
Intended for: ocular laser treatment procedures, including coagulation of abnormal retinal vasculature; and capturing real-time digital images of the anterior/posterior eye segments created using colour, fluorescein angiography and infrared imaging, for diagnosis/treatment planning.
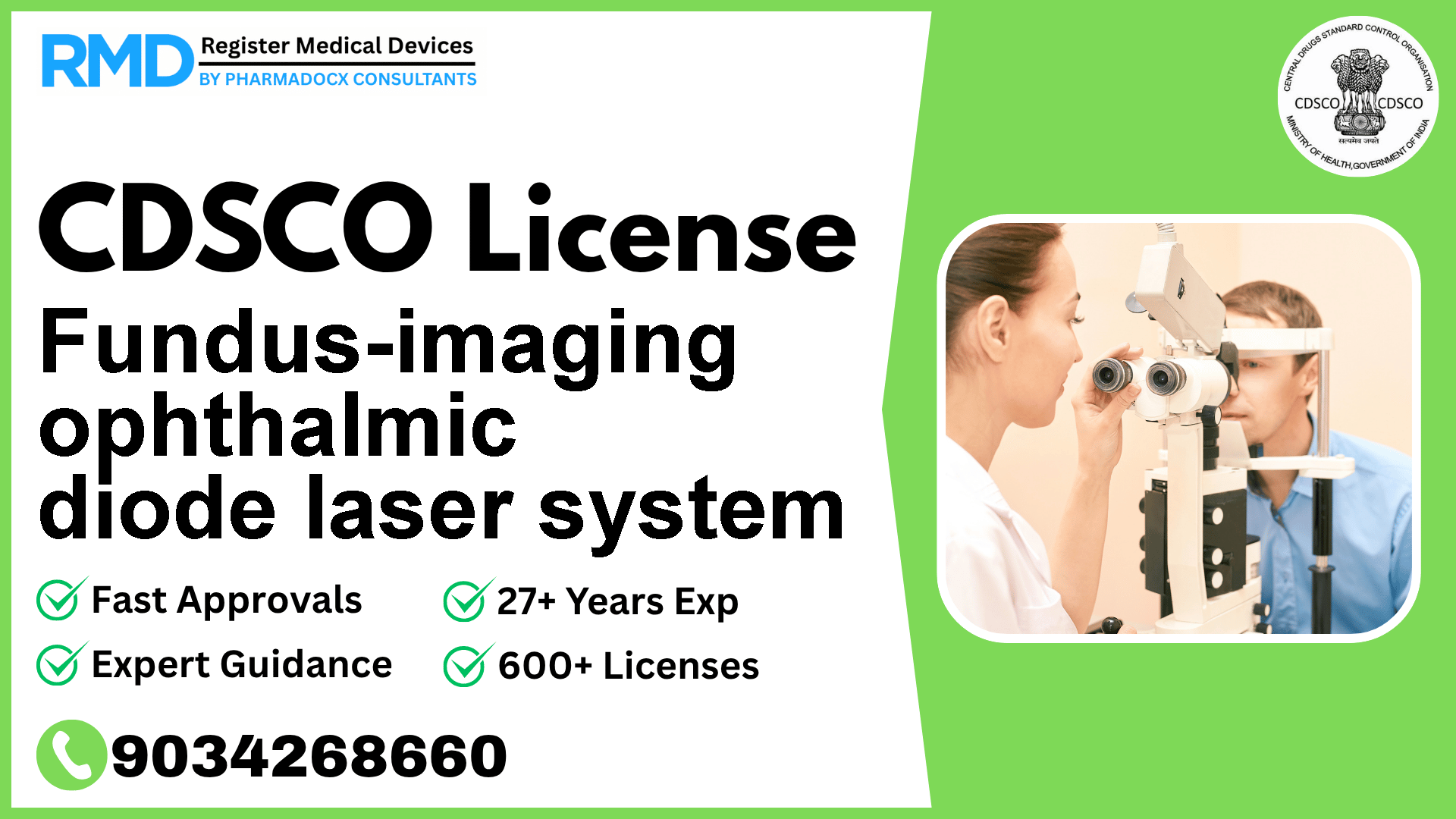
Comprehensive Guide to CDSCO Licensing for Fundus-Imaging Ophthalmic Diode Laser System (Class C)
As specialists with over 25 years of experience in India's medical device regulatory landscape, we have assisted more than 500 manufacturers and importers in successfully obtaining CDSCO licenses. In this guide, we focus on the Fundus-imaging ophthalmic diode laser system, a Class C medical device used in ophthalmology for laser treatment and diagnostic imaging.
Introduction: Device Overview and Regulatory Importance
The Fundus-imaging ophthalmic diode laser system is essential for ocular laser procedures such as coagulation of abnormal retinal vasculature and capturing real-time digital images of eye segments using colour, fluorescein angiography, and infrared imaging. Given its critical role in diagnosis and treatment planning, it is categorized as a Class C device under CDSCO regulation. Proper licensing ensures compliance with Indian safety and efficacy standards, enabling manufacturers and importers to legally market this advanced technology in India.
CDSCO Regulatory Framework for Fundus-Imaging Ophthalmic Diode Laser Systems
The Central Drugs Standard Control Organisation (CDSCO) regulates medical devices under the Medical Device Rules (MDR), 2017. Devices in Class C, such as the Fundus-imaging ophthalmic diode laser system, require a Manufacturing License (MD9) or Import License (MD15) from the Central Licensing Authority. Our role as regulatory consultants is to guide you through this stringent process efficiently.
Risk Classification and License Requirements
| Device Type | Risk Class | License Type | Licensing Authority | Typical Timeline | Government Fees |
|---|---|---|---|---|---|
| Fundus-Imaging Ophthalmic Diode Laser System | C | MD9 (Manufacturing) / MD15 (Import) | Central Licensing Authority | 4-5 months (MD9) / 5-6 months (MD15) | Rs 50,000 + Rs 1,000 per product (MD9) / USD 3,000 + USD 1,500 per product (MD15) |
You can verify the device classification on our Medical Device Classification page.
Manufacturing License Process (MD9) for Class C Devices
The MD9 license is mandatory for manufacturers intending to produce Fundus-imaging ophthalmic diode laser systems in India. The process involves:
- Test License (MD13): Apply for a test license to manufacture prototype batches for testing. This takes approximately 1.5 to 2 months.
- Product Testing: Conduct mandatory testing at CDSCO-approved government laboratories. Check the list of testing laboratories to choose an approved facility.
- Document Preparation: Compile all required documents, including the Device Master File, Plant Master File, Risk Management File, and Essential Principles Checklist.
- Apply for MD9 License (Form MD7): Submit the application on the CDSCO MD Online Portal along with the required documents and fees.
- Audit: CDSCO inspectors conduct a detailed audit of the manufacturing facility, quality management system (QMS), and technical documentation.
- Queries and Clarifications: Respond promptly to any queries raised by the CDSCO or audit team to avoid delays.
- Grant of License: Upon satisfactory review and compliance, the MD9 license is granted.
For a detailed walkthrough, our MD9 License Guide provides step-by-step assistance.
Manufacturing License Documents Required
Accurate and complete documentation is critical. For Class C devices like the Fundus-imaging ophthalmic diode laser system, you must prepare:
- Company Constitution documents (e.g., Incorporation Certificate)
- Proof of ownership or lease agreement for manufacturing premises
- Technical staff qualifications and experience certificates
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing design and manufacturing processes (Guide to DMF)
- Plant Master File (PMF) describing facility layout and equipment (PMF Guide)
- Essential Principles Checklist confirming compliance with safety and performance standards
- Risk Management File demonstrating hazard identification and mitigation (Risk Management Guide)
- Government-approved test reports from certified laboratories
- Labels and Instructions for Use (IFU) conforming to regulatory requirements
- Quality Management System (QMS) documentation, preferably ISO 13485:2016 certification
Import License Process (MD15) for Fundus-Imaging Ophthalmic Diode Laser Systems
If you are an importer, the MD15 license is mandatory. The import process involves:
- Document Preparation: Assemble required documents including manufacturing license from the country of origin, CE certificates, ISO 13485, Free Sale Certificates, device and plant master files.
- Application Submission: File Form MD14 via the CDSCO MD Online Portal with all supporting documents.
- Evaluation and Queries: CDSCO reviews the application and raises queries if necessary.
- Grant of License: License is issued on Form MD15 after successful evaluation.
The import license process typically takes 5 to 6 months. Refer to our detailed Import License Guide for in-depth information.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate issued by the regulatory authority of the exporting country
- ISO 13485:2016 certification for quality management
- CE Certificate or equivalent conformity assessment
- Device Master File and Plant Master File
- Wholesale License (if applicable)
- Company Constitution documents
Timeline and Processing Duration
| Stage | Duration |
|---|---|
| Test License (MD13) | 1.5 to 2 months |
| Product Testing | 1 to 1.5 months |
| Document Preparation | 2 to 3 weeks |
| License Application (MD9) | 4 to 5 months total |
The entire manufacturing license process typically spans 4 to 5 months, including testing and audits. Import licenses (MD15) take about 5 to 6 months.
Government Fees and Costs
| License Type | Application Fee | Per Product Fee | Total Estimated Cost* |
|---|---|---|---|
| MD9 License | Rs 50,000 | Rs 1,000 | Rs 51,000+ (depends on product count) |
| MD15 License | USD 3,000 | USD 1,500 | USD 4,500+ (varies by product) |
*Note: Additional costs include testing fees, notified body audit charges, and consultancy fees if applicable.
Common Challenges and Solutions
- Delayed Testing: Testing at government-approved labs can be backlogged. We recommend pre-booking slots early and considering alternate labs from the CDSCO Testing Laboratories list.
- Incomplete Documentation: Missing or inconsistent documents cause frequent application rejections. Engage experts to review your Device Master File and Risk Management File.
- Audit Non-Compliance: Facilities often fail to meet audit criteria due to inadequate QMS implementation. Conduct internal audits prior to CDSCO inspection.
- Regulatory Updates: The MDR framework is evolving. Stay updated by regularly checking notifications like the Fundus-imaging ophthalmic diode laser system notification Fts No. 29/MiscJO3/2020-DC (187).
Expert Consultation and Support
Navigating the CDSCO licensing process for Class C devices requires meticulous planning and expert knowledge. Our consultancy offers:
- Comprehensive gap analysis of your current compliance status
- End-to-end documentation preparation and review
- Liaison with notified bodies and CDSCO officials
- Training on QMS and risk management implementation
- Post-license surveillance and regulatory updates
Our proven track record with over 500 successful projects underscores our commitment to your smooth market entry.
Getting Started with Your CDSCO License Application
To initiate your licensing journey for the Fundus-imaging ophthalmic diode laser system:
- Assess Your Device Classification: Confirm Class C status using the CDSCO classification tool.
- Plan for Test License (MD13): Submit your test license application early to avoid bottlenecks.
- Engage an Approved Testing Lab: Choose from the official Testing Laboratories list.
- Prepare Your Documentation: Develop detailed Device and Plant Master Files, Risk Management, and QMS documents.
- Submit Your Application: Use the CDSCO MD Online Portal for all filings.
- Schedule Facility Audit: Coordinate with CDSCO for timely inspections.
- Respond to Queries Promptly: Ensure swift replies to avoid delays.
By following these actionable steps and leveraging our expertise, you can confidently navigate the CDSCO licensing pathway and successfully launch your Fundus-imaging ophthalmic diode laser system in the Indian market.
For a personalized consultation or to get started, contact our regulatory experts today and benefit from our 25+ years of industry experience.