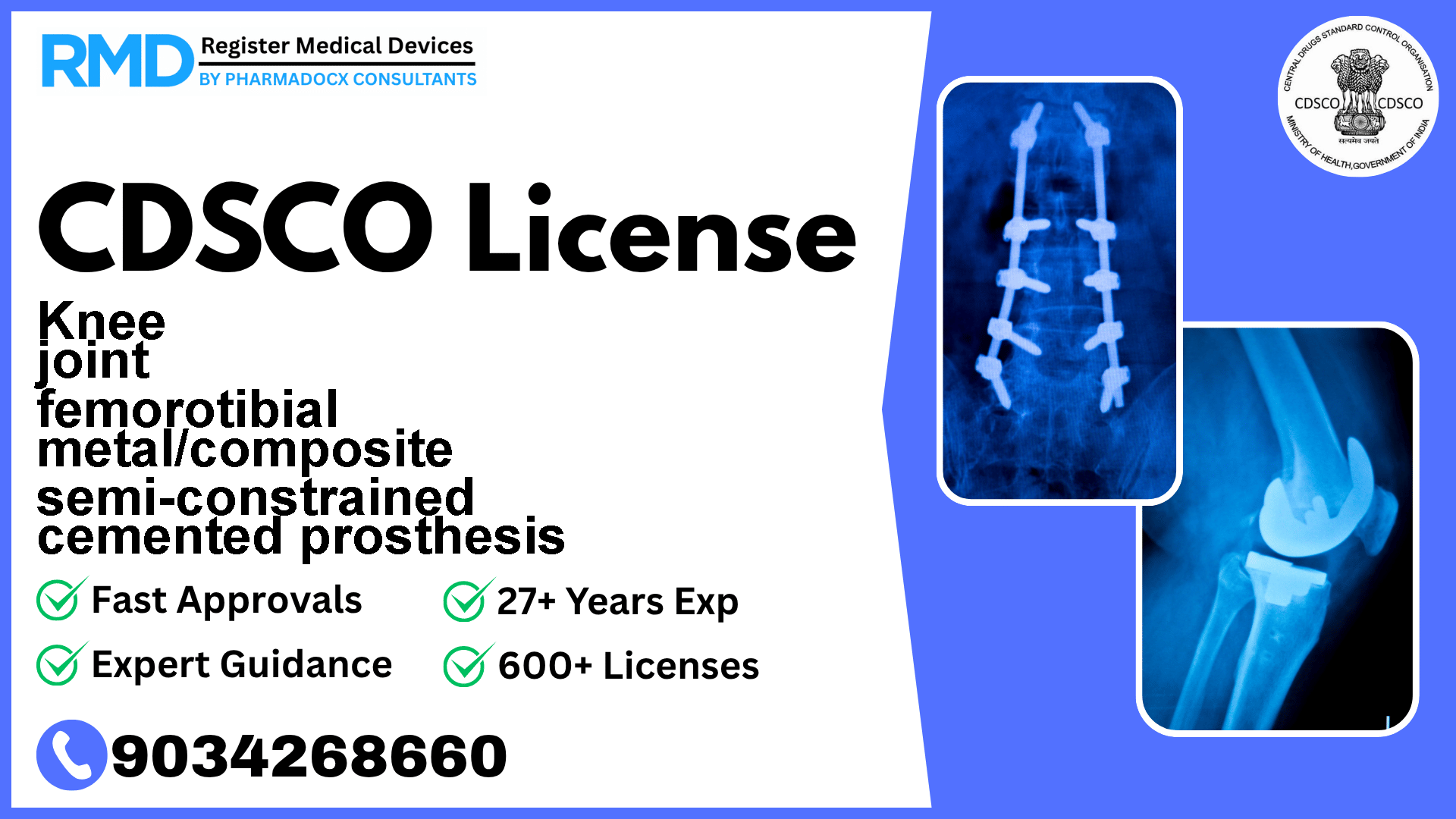
CDSCO License for Knee joint femorotibial metal/composite semi-constrained cemented prosthesis
Medical Device Information
Intended Use
Intended to be implanted to replace a hip joint
Comprehensive Guide to CDSCO Licensing for Knee Joint Femorotibial Semi-Constrained Cemented Prosthesis (Class C Orthopaedic Implant)
Navigating the regulatory landscape for medical devices in India can be challenging, especially for critical orthopaedic implants like the Knee Joint Femorotibial Metal/Composite Semi-Constrained Cemented Prosthesis. This device, classified as Class C under the CDSCO framework, requires stringent compliance due to its invasive nature and the high-risk profile associated with implantable devices.
With over 25 years of industry experience and having supported more than 500 companies in securing their CDSCO licenses, we provide you with an actionable, detailed roadmap to successfully launch your knee joint prosthesis in the Indian market.
CDSCO Regulatory Framework for Knee Joint Femorotibial Prosthesis
The Central Drugs Standard Control Organization (CDSCO) governs the approval and licensing of medical devices in India. As an orthopaedic implant intended to replace the hip joint, your knee joint femorotibial prosthesis falls under the notified category via Notification 29/Misc/3/2017-DC (292), dated 06.06.2018.
Given its classification as a Class C medical device, regulatory oversight is handled by the Central Licensing Authority, ensuring higher safety and efficacy standards are met before market entry.
Risk Classification and License Requirements
Risk Class: C
Class C devices are moderate to high-risk implants requiring a central approval process. This means manufacturers must apply for the MD9 Manufacturing License and importers require the MD15 Import License.
- MD9 License (Form MD7): For manufacturing Class C devices, granted by the CDSCO Central Licensing Authority.
- MD15 License (Form MD14): For importing Class C devices, also under the jurisdiction of the Central Licensing Authority.
Understanding this classification is critical to preparing the right documentation, undergoing mandatory audits, and ensuring compliance with Indian regulations.
For a detailed overview of device classification, you can refer to our Medical Device Classification guide.
Manufacturing License Process (MD9) for Class C Devices
The MD9 license process is comprehensive and generally takes 4 to 5 months from start to finish. The key stages include:
- Test License Application (Form MD13): Mandatory initial step, valid for 6 months, allowing you to conduct product testing.
- Product Testing: Conducted at CDSCO-approved laboratories to verify safety and performance.
- Document Preparation: Compilation of all required technical and quality documents.
- Application Submission (Form MD7): Formal manufacturing license application via the CDSCO MD Online Portal.
- CDSCO Inspection/Audit: CDSCO officials perform on-site audits to verify compliance.
- Query Resolution: Addressing any concerns or deficiencies flagged during inspection.
- Grant of MD9 License (Form MD9): Upon satisfactory review, the manufacturing license is issued.
Practical Tip:
Initiate the Test License process well in advance to accommodate possible delays in lab testing, which typically takes 1.5 to 2 months.
Manufacturing License Documents Required for MD9
The documentation for MD9 is extensive. Precise preparation is key to avoiding delays. Essential documents include:
- Company Constitution and Incorporation Certificate
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualification Certificates of Technical Staff
- Fire No Objection Certificate (NOC)
- Pollution Control Board NOC
- Device Master File (DMF): Detailed design, specifications, and manufacturing processes (DMF Guide)
- Plant Master File (PMF): Description of manufacturing facility and quality systems (PMF Guide)
- Essential Principles Checklist confirming compliance with regulatory standards
- Risk Management File demonstrating hazard analysis and mitigation (Risk Management)
- Test Reports from CDSCO-approved laboratories (Testing Labs)
- Product Labels and Instructions for Use (IFU)
- Quality Management System documentation (typically ISO 13485:2016 compliant)
Attention to detail in these documents significantly enhances the likelihood of approval on first submission.
Import License Process (MD15) for Class C Devices
Importers of knee joint prostheses must secure the MD15 license, which typically requires 5 to 6 months. The process steps are:
- Document Preparation: Assemble all required certificates and licenses.
- Application Submission (Form MD14): Submit via the CDSCO MD Online Portal.
- Review and Query Resolution: Respond promptly to any CDSCO inquiries.
- Grant of MD15 License: Upon successful evaluation, the import license is issued.
Notably, no test license is required for import applications.
Import License Documents Required for MD15
Key documents include:
- Valid Manufacturing License (MD9 if Indian manufacturer or equivalent foreign license)
- Free Sale Certificate from the country of origin
- ISO 13485:2016 Certification
- CE Certificate or equivalent international certification
- Device Master File and Plant Master File
- Wholesale Drug License (if applicable)
- Company Constitution and Registration Proof
Ensuring these documents are complete and authentic helps smooth the approval process.
Timeline and Processing Duration
| License Type | Typical Duration |
|---|---|
| MD9 Manufacturing | 4 to 5 months |
| MD15 Import | 5 to 6 months |
| Test License (MD13) | 1.5 to 2 months |
Allow extra time for audit scheduling and potential query resolution. Early and thorough preparation is essential.
Government Fees and Costs
| License Type | Application Fee | Per Product Fee |
|---|---|---|
| MD9 Manufacturing | INR 50,000 | INR 1,000 |
| MD15 Import | USD 3,000 (approx. INR 2.4 Lakh) | USD 1,500 (approx. INR 1.2 Lakh) |
Fees must be paid online via the CDSCO portal during application submission.
Common Challenges and Practical Solutions
- Delayed Testing Results: Partner with CDSCO-recognized labs early and confirm their capacity and turnaround times.
- Incomplete Documentation: Conduct internal audits against CDSCO checklists before submission.
- Audit Non-compliance: Prepare your facility with mock audits and train staff on regulatory expectations.
- Query Delays: Assign a dedicated regulatory liaison to respond promptly to CDSCO communications.
Our experience shows that manufacturers who proactively engage with notified bodies and CDSCO inspectors can significantly reduce approval timelines.
Expert Consultation and Support
Navigating CDSCO's complex regulatory pathways requires expertise. Our team offers:
- Comprehensive document preparation services, including DMF and PMF development
- Coordination with CDSCO-approved testing laboratories
- Pre-audit readiness assessments and mock inspections
- End-to-end application submission and query management
We leverage our 25+ years of experience and a proven track record of 500+ successful client approvals to provide tailored, actionable guidance.
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm your knee joint prosthesis as Class C.
- Prepare Test License (MD13): Initiate this to begin product testing.
- Compile Required Documentation: Use our Device and Plant Master File guides and Plant Master File guide for detailed templates.
- Submit Test License Application: Via the CDSCO MD Online Portal.
- Coordinate Product Testing: Engage approved laboratories early.
- Prepare MD9 Manufacturing License Application: Once test results are in, compile final documents.
- Request Audit: Coordinate with CDSCO for inspection scheduling.
- Submit MD9 Application: Through the CDSCO portal, monitor progress.
For importers, parallelly prepare MD15 documentation and submit after manufacturing license confirmation.
By adhering to this roadmap and leveraging expert assistance, you can confidently bring your knee joint femorotibial prosthesis to the Indian market while ensuring full regulatory compliance.
Feel free to contact us for a personalized consultation to start your CDSCO licensing journey.