CDSCO License for Knee joint tibial (hemi-knee) metallic resurfacing uncemented prosthesis
Medical Device Information
Intended Use
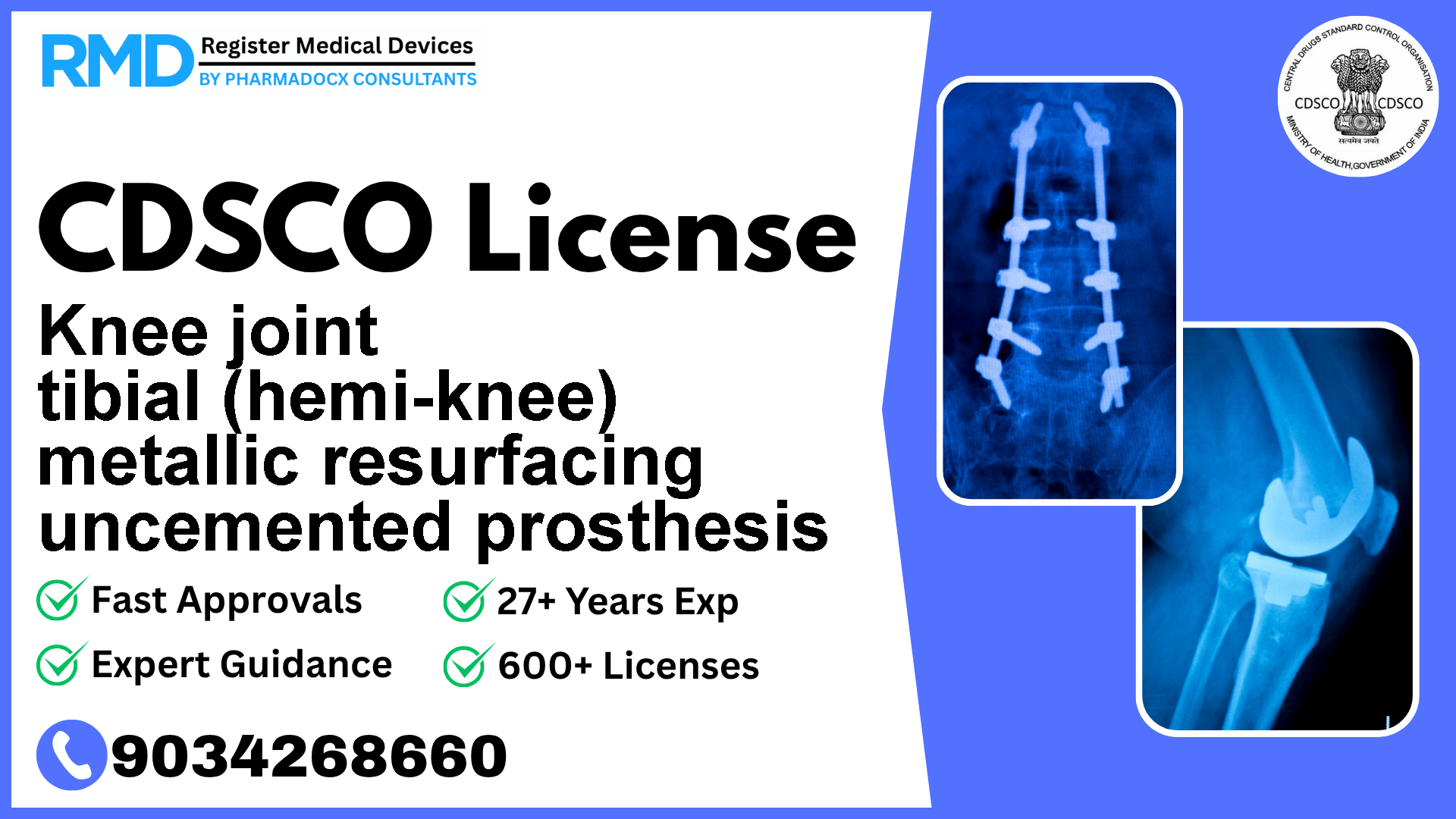
Intended to be implanted to replace part of a knee joint
Comprehensive Guide to CDSCO Licensing for Knee Joint Tibial (Hemi-Knee) Metallic Resurfacing Uncemented Prosthesis
As seasoned regulatory consultants with over 25 years of experience and having assisted 500+ manufacturers and importers, we understand the complexities involved in navigating the CDSCO licensing landscape. This guide focuses specifically on the Knee Joint Tibial (hemi-knee) metallic resurfacing uncemented prosthesis, a Class C orthopaedic implant, outlining the regulatory framework, license requirements, timelines, costs, and practical steps to facilitate your entry into the Indian medical device market.
Understanding Your Device and Its Regulatory Importance
The knee joint tibial (hemi-knee) metallic resurfacing uncemented prosthesis is an implant intended to replace part of the knee joint. Given its invasive nature and direct implantation, it falls under Class C risk category, indicating a moderate to high risk. Proper regulatory compliance ensures patient safety, product efficacy, and market authorization, making CDSCO licensing an essential milestone.
CDSCO Regulatory Framework for Orthopaedic Implants (Class C Devices)
The Central Drugs Standard Control Organization (CDSCO) regulates all medical devices in India under the Medical Device Rules (MDR) 2017. Orthopaedic implants like the knee prosthesis are classified as Class C devices due to their implantable nature and risk profile.
For Class C devices, the MD9 manufacturing license is mandatory for domestic manufacturers, and the MD15 import license is required for importers. This ensures rigorous evaluation including product testing, factory inspections, and compliance with essential principles.
Risk Classification and License Requirements for Knee Joint Prosthesis
- Device Risk Class: Class C
- License Type for Manufacturing: MD9 License (via Form MD7)
- License Type for Import: MD15 License (via Form MD14)
- Authority: Central Licensing Authority, CDSCO
Class C devices require a detailed audit and testing process, including a mandatory test license (MD13) prior to full manufacturing license application.
Manufacturing License Process (MD9) for Class C Knee Prosthesis
Test License Application (Form MD13):
- Duration: Approximately 1.5 to 2 months
- Purpose: Allows initial manufacturing and sample testing
Product Testing:
- Conducted at CDSCO-approved government laboratories
- Testing labs list is available on the CDSCO Testing Laboratories portal
Document Preparation:
- Compile the Device Master File (DMF), Plant Master File (PMF), Risk Management File, Essential Principles Checklist, and Quality Management System (QMS) documentation
License Application Submission (Form MD7):
- Submit through the CDSCO MD Online Portal
Audit by CDSCO Inspectors:
- Comprehensive inspection of manufacturing premises and QMS implementation
Resolution of Queries:
- Promptly address any clarifications or observations raised during audit
Grant of MD9 License:
- Upon satisfactory assessment, license issued on Form MD9
For detailed guidance, refer to our MD9 License Guide.
Manufacturing License Documents Required for MD9
- Company Constitution (MOA, AOA, Partnership Deed)
- Proof of Ownership or Lease of Manufacturing Premises
- Details and qualifications of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) – design, materials, manufacturing processes
- Plant Master File (PMF) – facility layout, manufacturing workflow
- Essential Principles Checklist confirming compliance with MDR
- Risk Management File aligned with ISO 14971
- Test Reports from approved laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System Documents (ISO 13485:2016 certification recommended)
Our comprehensive Device Master File guide and Plant Master File guide will help you prepare these critical documents efficiently.
Import License Process (MD15) for Knee Joint Prosthesis
Unlike manufacturing, importers do not require a test license. The MD15 import license process includes:
Document Preparation:
- Manufacturing license from the country of origin
- Free Sale Certificate
- ISO 13485:2016 and CE Certificate
- Device and Plant Master Files
- Wholesale License (if applicable)
- Company Constitution
Application Submission:
- File application on CDSCO MD Online Portal
Queries Resolution:
- Address any departmental queries promptly
License Grant:
- Upon clearance, MD15 license is issued
For a detailed walkthrough, visit our Import License Guide.
Import License Documents Required
- Manufacturing License of the Product from Country of Origin
- Free Sale Certificate
- ISO 13485:2016 Certification
- CE Certificate (if applicable)
- Device Master File
- Plant Master File
- Wholesale License
- Company Constitution and Board Resolution
Timeline and Processing Duration
| Process Step | Estimated Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 3 – 4 weeks |
| Document Preparation | 4 – 6 weeks (overlapping) |
| License Application Processing | 2 – 3 months |
| Total Time for MD9 License | Approximately 4 – 5 months |
| Total Time for MD15 Import License | Approximately 5 – 6 months |
Government Fees and Costs
| License Type | Application Fee (INR) | Per Product Fee (INR) |
|---|---|---|
| MD9 (Class C) | 50,000 | 1,000 |
| MD15 Import | USD 3,000 (approx) | USD 1,500 (approx) |
Note: Fees are payable online through the CDSCO portal. Additional costs include laboratory testing fees and audit charges which vary by notified bodies.
Common Challenges and Solutions
Challenge: Delays in product testing due to limited lab slots.
- Solution: Engage with CDSCO-accredited labs early; refer to the Testing Laboratories list.
Challenge: Incomplete documentation causing audit observations.
- Solution: Use comprehensive checklists and templates for Device Master File and Risk Management, available in our guides.
Challenge: Non-compliance with Essential Principles.
- Solution: Implement a robust Quality Management System aligned with ISO 13485 and regularly update risk management per Risk Management best practices.
Challenge: Coordination between multiple stakeholders (manufacturers, labs, notified bodies).
- Solution: Appoint a dedicated regulatory project manager to streamline communication and timelines.
Expert Consultation and Support
Navigating CDSCO licensing for Class C devices like the knee joint tibial prosthesis requires expertise and precision. Our team has successfully guided over 500 companies through this process, ensuring timely approvals and compliance. We provide end-to-end support including document preparation, audit readiness, lab coordination, and application submission.
Getting Started with Your CDSCO License Application
Assess your device classification: Confirm Class C status using the Medical Device Classification resource.
Initiate documentation: Begin compiling your Device Master File and Plant Master File with our detailed guides.
Apply for test license (MD13): Submit your test license application via the CDSCO MD Online Portal.
Schedule product testing: Engage with CDSCO-approved labs early to avoid delays.
Prepare for audit: Ensure your manufacturing facility and QMS are inspection-ready.
Submit manufacturing license application (Form MD7): Complete the online application once testing and audit requirements are met.
Monitor and respond promptly: Keep track of application status and respond to queries quickly to prevent processing delays.
By following these actionable steps, manufacturers and importers of knee joint tibial metallic resurfacing prostheses can confidently navigate the CDSCO licensing process and successfully enter the Indian market.
For personalized assistance and to accelerate your regulatory journey, contact our expert consultants today.