CDSCO License for Powered X-rays radiation therapy table
Medical Device Information
Intended Use
A programmable electrically operated bed for radiotherapy designed to adjust the patient's posture and immobilize the patient for treatment that uses an X- ray therapy apparatus.
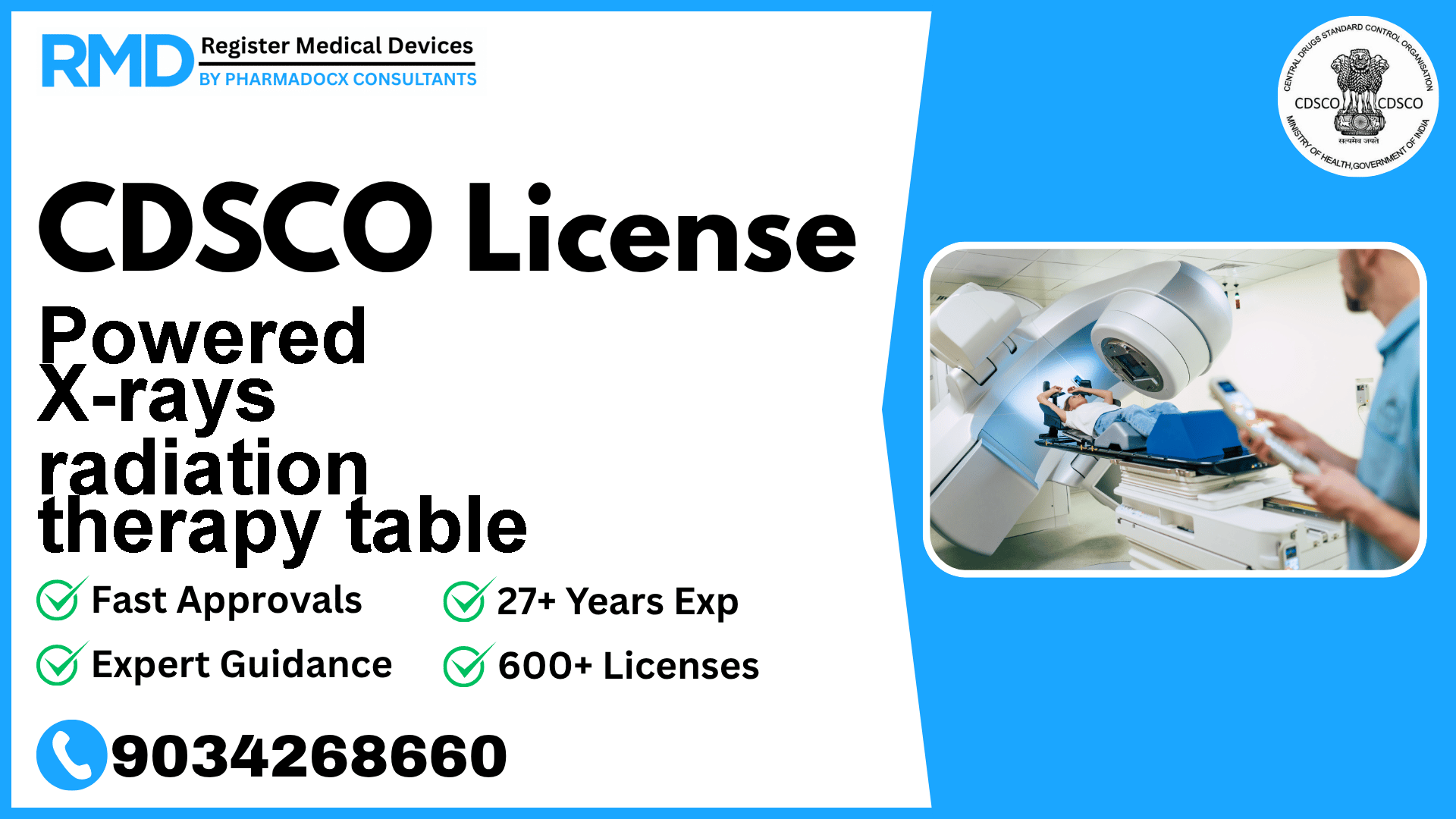
Comprehensive Guide to CDSCO Licensing for Powered X-rays Radiation Therapy Table (Class B Device)
Introduction: Understanding the Powered X-rays Radiation Therapy Table and Its Regulatory Significance
Powered X-rays radiation therapy tables are critical medical devices used in radiotherapy to precisely position and immobilize patients during treatment. As electrically operated programmable beds tailored for X-ray therapy applications, these devices fall under the Class B risk category according to CDSCO’s classification. Given their vital role in cancer treatment and patient safety implications, obtaining the appropriate CDSCO manufacturing license is mandatory for manufacturers seeking to enter the Indian market.
With over 25 years of experience, we have assisted 500+ companies in navigating the CDSCO licensing landscape, ensuring timely approvals and compliance. In this guide, we delve into the regulatory framework, process specifics, and practical tips tailored for Powered X-rays radiation therapy tables.
CDSCO Regulatory Framework for Radiotherapy Devices
The Central Drugs Standard Control Organisation (CDSCO) governs the import and manufacture of medical devices in India. The regulatory framework mandates that Class B devices like the Powered X-rays radiation therapy table require a manufacturing license under Form MD5, issued by the State Licensing Authority.
This framework ensures compliance with safety, quality, and performance standards through rigorous documentation, product testing, and audits. The notification governing this device type is File No. 29/Misc./03/2020-DC (180), dated 6.8.2021, specifically categorizing radiotherapy devices including programmable therapy tables.
Risk Classification and License Requirements for Powered X-rays Radiation Therapy Tables
Powered X-rays radiation therapy tables are classified as Class B devices, indicating a low-moderate risk profile. Consequently, manufacturers must obtain the MD5 license (per CDSCO guidelines):
- License Type: MD5 (Manufacturing License for Class A and B devices)
- Authority: State Licensing Authority
- Application Form: MD3 for license application; MD13 for test license
- Total Processing Time: Approximately 3-4 months
- Applicable Fees: Rs 5,000 per application + Rs 500 per product
This license authorizes the manufacturing of Powered X-rays radiation therapy tables within the state and requires compliance with both central and state regulations.
Manufacturing License Process (MD5) for Powered X-rays Radiation Therapy Table
The MD5 licensing process involves several key steps:
- Test License Application (Form MD13): Initiate by applying for a test license to legally manufacture the device for testing purposes. This stage takes about 1.5-2 months.
- Product Testing: Conduct mandatory tests at CDSCO-approved laboratories to verify compliance with essential principles and safety standards. Refer to the list of testing laboratories.
- Documentation Preparation: Compile comprehensive documentation including Device Master File, Plant Master File, Risk Management File, and QMS documents.
- License Application (Form MD3): Submit the manufacturing license application through the CDSCO MD Online Portal.
- Audit by Notified Body: Undergo an audit by a CDSCO-approved notified body listed here to assess manufacturing site compliance.
- Resolution of Queries: Address any observations or queries raised by the licensing authority or auditors.
- Grant of License (Form MD5): Upon satisfactory review, the license is granted allowing commercial manufacturing.
Manufacturing License Documents Required for MD5 Application
For your Powered X-rays radiation therapy table, the following documents are essential:
- Company Constitution Documents: Certificate of Incorporation, Memorandum & Articles of Association
- Proof of Premises Ownership or Lease Agreement
- Technical Staff Qualification and Experience Certificates
- Fire and Pollution NOCs: To comply with safety and environmental regulations
- Device Master File (DMF): Detailed device design, specifications, and manufacturing process (Learn more)
- Plant Master File (PMF): Description of manufacturing facility, quality control systems (Guide here)
- Essential Principles Checklist: Evidence of conformity with Indian Medical Device Rules
- Risk Management File: Documented risk analysis and mitigation strategies (Implementation tips)
- Test Reports: From CDSCO-approved labs validating safety and performance
- Labels and Instructions for Use (IFU): Compliant with regulatory standards
- Quality Management System (QMS) Documents: ISO 13485 certification and related SOPs
Import License Process (MD15) for Powered X-rays Radiation Therapy Tables
If you are an importer of Powered X-rays radiation therapy tables, the MD15 license is mandatory:
- Authority: Central Licensing Authority
- Application Form: MD14 for license application
- Timeline: Approximately 5-6 months
- Documents Required: Manufacturing license from country of origin, Free Sale Certificate, ISO 13485:2016 certification, CE certificate if applicable, Device Master File, Plant Master File, wholesale license, and company constitution documents.
The import license application is also submitted through the CDSCO MD Online Portal.
Import License Documents Required for MD15 Application
Ensure you compile the following:
- Valid manufacturing license from the exporting country
- Free Sale Certificate or equivalent regulatory approval from the country of origin
- ISO 13485:2016 Quality Management System certificate
- CE Certificate (if applicable)
- Detailed Device Master File and Plant Master File
- Wholesale drug license (if applicable)
- Company Constitution and legal documents
Timeline and Processing Duration Breakdown
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 2 - 3 weeks |
| Document Preparation | 2 - 3 weeks |
| License Application (MD3) | Immediate submission |
| Audit by Notified Body | 3 - 4 weeks |
| Query Resolution | 2 - 3 weeks |
| Final License Issuance (MD5) | Total ~3-4 months |
Government Fees and Cost Structure
For your Class B Powered X-rays radiation therapy table manufacturing license:
- Application Fee: Rs 5,000 per application
- Per Product Fee: Rs 500
Additional costs include testing laboratory fees (varies by lab and tests required) and notified body audit charges (approx Rs 1,00,000 - Rs 1,50,000 depending on scope).
Common Challenges and Practical Solutions
- Delayed Test Report Availability: Coordinate early with approved laboratories to book testing slots in advance.
- Incomplete Documentation: Use detailed checklists for DMF, PMF, and Risk Management files to avoid back-and-forth with CDSCO.
- Audit Non-Compliance: Engage experienced consultants to pre-audit your manufacturing site and QMS.
- Query Resolution Delays: Assign a dedicated regulatory liaison to promptly handle CDSCO queries.
Our experience shows proactive planning and expert document management significantly reduce approval timelines.
Expert Consultation and Support for CDSCO Licensing
Navigating the CDSCO licensing process can be complex, especially for specialized devices like Powered X-rays radiation therapy tables. We offer end-to-end consultancy services including:
- Gap assessment and compliance roadmap
- Preparation of Device and Plant Master Files
- Risk management documentation
- Coordination with testing labs and notified bodies
- Application filing and follow-up
Our track record of assisting 500+ companies ensures your application stands the best chance of success.
Getting Started with Your CDSCO License Application
- Evaluate Device Classification: Confirm Class B classification per the latest CDSCO notifications.
- Prepare Technical and Quality Documents: Start compiling the DMF, PMF, risk files, and QMS documentation.
- Apply for Test License (MD13): Submit your test license application via the CDSCO MD Online Portal.
- Coordinate Product Testing: Book slots at CDSCO-approved labs early.
- Engage a Notified Body: Identify and schedule audits with a notified body from the official list.
- Submit Manufacturing License Application (MD3): Once testing and documentation are ready.
- Prepare for Audit and Query Resolution: Be ready with all records and compliance evidence.
Starting early and following a structured approach can reduce your licensing time by weeks. Contact us today to leverage our comprehensive expertise in securing your MD5 license for Powered X-rays radiation therapy tables.