CDSCO License for Ophthalmic calliper
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
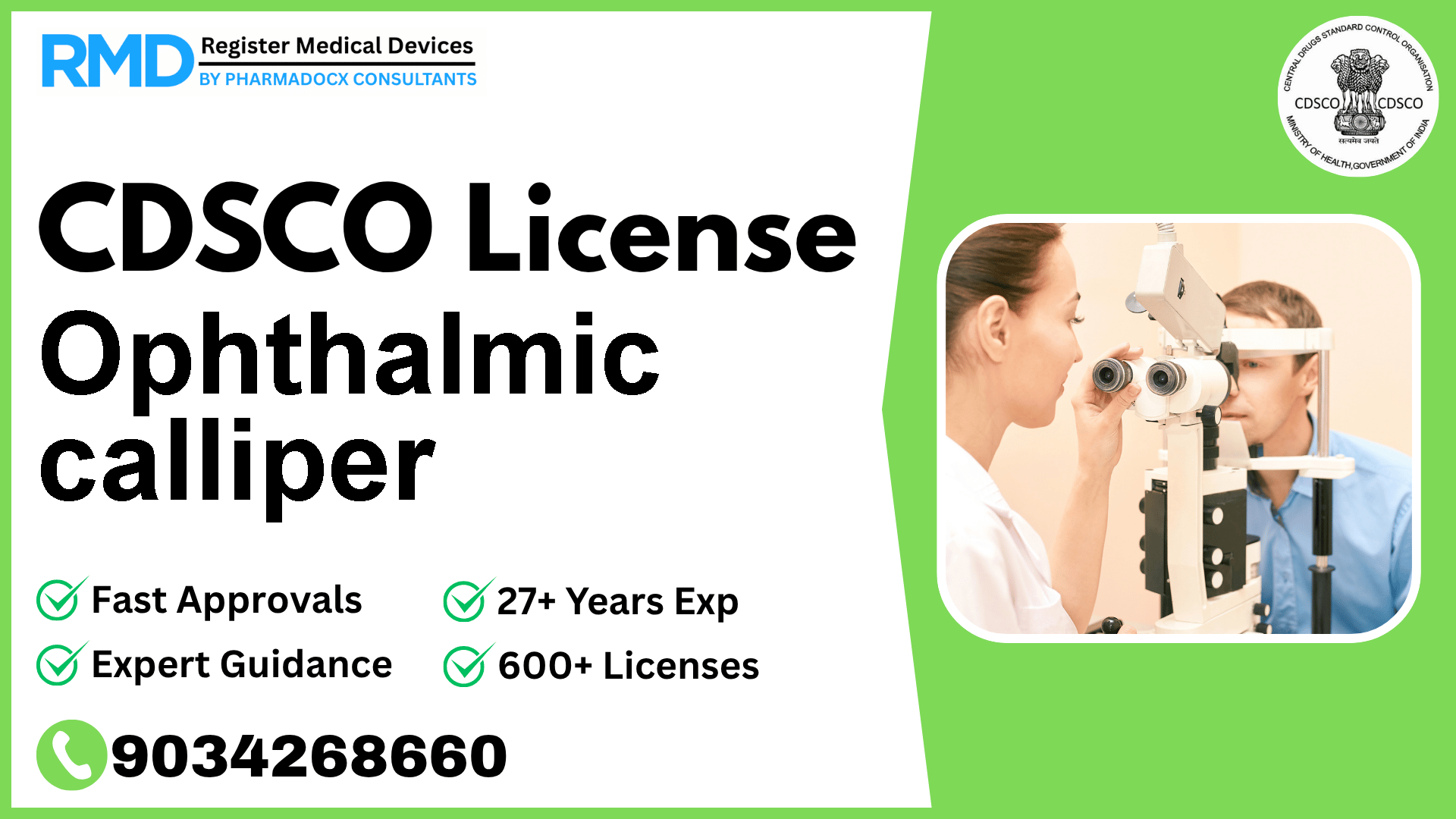
A hand-held manual ophthalmic measuring instrument consisting of two legs hinged at one end and designed to measure the diameter, length, angles, and thicknesses of the eye.
Comprehensive Guide to CDSCO Licensing for Ophthalmic Callipers (Class A Medical Device)
At our firm, with over 25 years of expertise and having assisted more than 500 companies in securing CDSCO licenses, we understand the nuances and regulatory intricacies manufacturers and importers face with Class A medical devices like the Ophthalmic Calliper. This hand-held ophthalmic measuring instrument, used for precise measurement of eye parameters, falls under risk Class A as per CDSCO regulations.
Understanding the Ophthalmic Calliper and Regulatory Importance
The Ophthalmic Calliper is a precision manual instrument designed with two hinged legs to measure diameters, lengths, angles, and thicknesses of the eye. Given its direct medical application in ophthalmology, regulatory approval from CDSCO (Central Drugs Standard Control Organization) ensures the device meets safety and performance standards required by Indian law, particularly under the notification Fts No. 29/MiscJO3/2020-DC(187) dated 9.8.2021.
Ensuring compliance not only facilitates smooth market entry but also builds trust among healthcare providers and end-users.
CDSCO Regulatory Framework for Ophthalmic Callipers
Medical devices in India are regulated under the Medical Device Rules 2017, enforced by CDSCO. Ophthalmic Callipers are classified as Class A devices, the lowest risk category, but still subject to stringent requirements including manufacturing licensing, quality management systems, and product testing.
For Class A devices, the manufacturing license is granted by the State Licensing Authority via the MD5 license process. Importers of such devices require an MD15 import license from the central authority.
Risk Classification and License Requirements for Ophthalmic Callipers
- Risk Class: A (Low risk)
- License Type for Manufacturing: MD5 License (Application Form MD3)
- Regulatory Authority: State Licensing Authority
- Test License Required: Yes, MD13 Test License prior to manufacturing license application
- Audit Requirement: Yes, by a notified body
For detailed classification guidelines, you can review the Medical Device Classification resource.
MD5 Manufacturing License Process for Ophthalmic Callipers
Obtaining an MD5 license involves several key steps:
Apply for Test License (Form MD13): This preliminary license permits product testing and manufacturing for test purposes. It typically takes 1.5 to 2 months to obtain.
Product Testing: Conduct testing of the ophthalmic calliper in CDSCO-approved government laboratories. Refer to the Testing Laboratories List for authorized labs.
Documentation Preparation: Compile all necessary documentation, including Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, test reports, labels, Instructions for Use (IFU), and Quality Management System (QMS) documents.
Submit Application for Manufacturing License (Form MD3): File your application via the CDSCO MD Online Portal.
Audit by Notified Body: An audit of your manufacturing facility and QMS will be conducted by notified bodies listed on the Notified Bodies List.
Resolve Queries: Address any observations or queries raised by CDSCO or the notified body promptly.
Grant of License (Form MD5): Upon successful audit and review, the MD5 license is issued.
Manufacturing License Documents Required for Ophthalmic Callipers
- Company Constitution (Incorporation Certificate, Partnership Deed, etc.)
- Proof of ownership or valid lease agreement of manufacturing premises
- Technical staff qualification and experience documents
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) – detailed product specifications and design (Device Master File Guide)
- Plant Master File (PMF) – production processes and facilities (Plant Master File Guide)
- Essential Principles Checklist ensuring compliance with safety and performance
- Risk Management File demonstrating hazard identification and mitigation (Risk Management)
- Test Reports from CDSCO-approved labs
- Labels and Instructions for Use (IFU)
- Quality Management System documents, typically ISO 13485:2016 compliant
Import License Process for Ophthalmic Callipers (MD15 License)
For importers intending to bring Ophthalmic Callipers into India, the MD15 license is required from the Central Licensing Authority. The process is as follows:
Document Preparation: Gather required documents such as the manufacturing license of the foreign manufacturer, Free Sale Certificate, ISO 13485:2016 certification, CE certificate (if applicable), Device and Plant Master Files, wholesale license, and company constitution.
License Application: File application using Form MD14 via the CDSCO MD Online Portal.
Query Resolution: Respond to any departmental queries.
License Grant (Form MD15): Upon satisfaction, CDSCO grants the import license.
Import License Documents Required
- Valid manufacturing license of the overseas manufacturer
- Free Sale Certificate from country of origin
- ISO 13485:2016 certification
- CE Certificate (if applicable)
- Device Master File
- Plant Master File
- Wholesale License (if applicable)
- Company Constitution documents
Timeline and Processing Duration
MD5 Manufacturing License: Approximately 3-4 months total
- Test License (MD13): 1.5-2 months
- Product Testing: 2-4 weeks
- Documentation and Application Processing: 1-2 months
- Audit and Query Resolution: 3-4 weeks
MD15 Import License: Approximately 5-6 months
Government Fees and Costs
MD5 License:
- Application Fee: ₹5,000
- Per Product Fee: ₹500
MD15 License:
- Class A Devices: USD 1,000 per site + USD 50 per product
Additional costs may include testing fees at government-approved labs and notified body audit charges.
Common Challenges and Solutions
- Delayed Test Reports: To avoid bottlenecks, schedule testing well in advance with CDSCO-approved labs.
- Incomplete Documentation: Utilize checklists and templates to ensure all documents like DMF and PMF are comprehensive and compliant.
- Audit Non-compliance: Engage with notified bodies early to understand audit expectations; conduct internal audits before official inspections.
- Query Resolution Delays: Assign a dedicated regulatory expert to respond promptly to CDSCO queries.
Expert Consultation and Support
Navigating CDSCO licensing requires detailed knowledge and experience. Our team has successfully guided over 500 clients through the MD5 and MD15 licensing processes, helping avoid common pitfalls and expediting approvals.
We offer:
- Comprehensive document preparation and review
- Pre-audit readiness assessments
- Liaison with notified bodies and CDSCO officials
- Guidance on product testing and compliance
Getting Started with Your CDSCO License Application for Ophthalmic Callipers
Assess your Device Class and Licensing Type: Confirm your device’s classification as Class A and prepare for the MD5 license.
Register on CDSCO MD Online Portal: Create your account and familiarize yourself with the portal interface.
Initiate Test License Application (Form MD13): Begin the process to secure the necessary test license.
Coordinate Product Testing: Contact CDSCO-approved labs early to schedule testing.
Prepare Device and Plant Master Files: Use expert templates and guides to ensure completeness.
Engage a Notified Body for Audit Preparation: Choose from the List of Notified Bodies to schedule your audit.
Submit Manufacturing License Application (Form MD3): Once test reports and documents are ready, apply via the CDSCO portal.
Plan for Query Management: Assign a team member or regulatory consultant to handle any follow-up communications.
For detailed assistance with each step, please reach out to our regulatory experts who can streamline your journey to compliance and market entry.
By following this structured approach, manufacturers and importers of Ophthalmic Callipers can confidently navigate the Indian regulatory landscape, ensuring timely approvals and successful commercialization.