CDSCO License for Ophthalmic head reflector
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
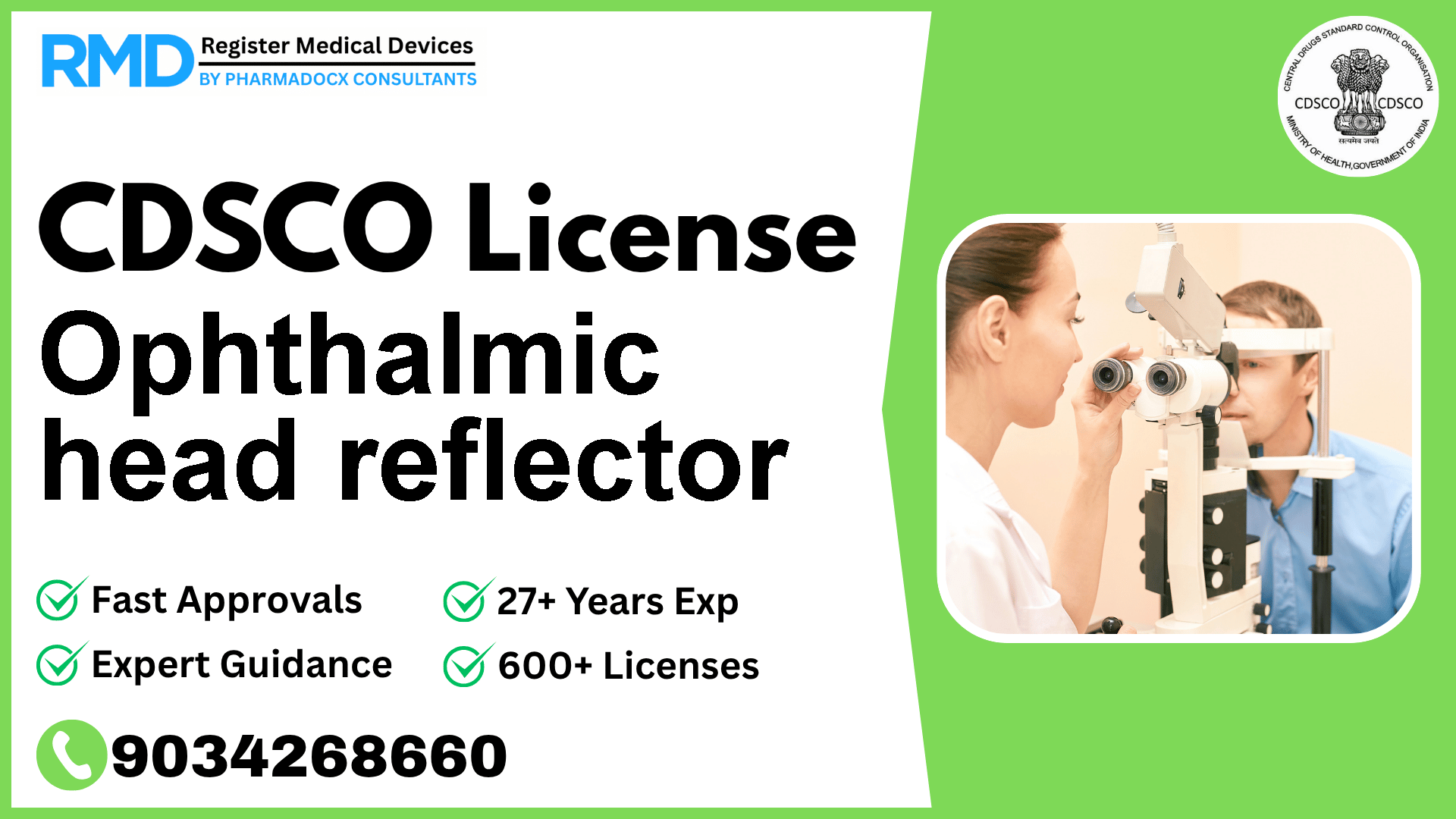
A head-worn ophthalmic device intended to reflect light onto the eye of a patient to allow examination of the eye and its associated structures.
Comprehensive Guide to CDSCO Licensing for Ophthalmic Head Reflector (Class A Medical Device)
The ophthalmic head reflector is a vital head-worn device designed to reflect light onto a patient's eye, facilitating detailed examination of the eye and its associated structures. Classified as a Class A medical device under Indian regulations, this device plays a crucial role in ophthalmology diagnostics. As regulatory consultants with over 25 years of experience and having aided 500+ companies, we provide you with a complete roadmap to secure your CDSCO license efficiently and compliantly.
CDSCO Regulatory Framework for Ophthalmic Head Reflector
The Central Drugs Standard Control Organization (CDSCO) governs medical device regulations in India. This device falls under the Class A risk category as per the Medical Device Classification, meaning it is considered low risk but still requires strict adherence to regulatory protocols before market entry.
The regulatory pathway involves obtaining a Manufacturing License (MD5) from the State Licensing Authority because Class A devices are regulated at the state level. The process also mandates a Test License (MD13) and product testing from government-approved laboratories.
Risk Classification and License Requirements
- Device: Ophthalmic Head Reflector
- Risk Class: A (Low Risk)
- License Type: MD5 (Manufacturing License)
- Authority: State Licensing Authority
- Notification Reference: Fts No. 29/MiscJO3/2020-DC (187), dated 9.8.2021
Being a Class A device, the compliance process is streamlined yet thorough, focusing on safety, efficacy, and quality management.
Manufacturing License Process (MD5) for Ophthalmic Head Reflector
Securing an MD5 license involves several methodical steps:
- Apply for Test License (Form MD13): The initial step is to apply for a test license which allows you to manufacture the device for testing purposes.
- Product Testing: Once the test license is granted, submit samples to one of the government-approved testing laboratories for analysis.
- Documentation Preparation: Simultaneously, prepare all necessary technical and administrative documents.
- Submit Application for Manufacturing License (Form MD3): After obtaining test results, apply for the MD5 license via the CDSCO MD Online Portal.
- Audit by Notified Body: A notified body from the official list will perform an audit of your manufacturing site and quality systems.
- Respond to Queries: Address any queries raised by the CDSCO or notified body promptly.
- Grant of License: Upon satisfactory compliance, the State Licensing Authority issues the MD5 license.
For detailed guidance on the MD5 license process, refer to our comprehensive MD5 License Guide.
Manufacturing License Documents Required for Ophthalmic Head Reflector
To ensure a smooth application process, the following documents must be prepared meticulously:
- Company Constitution Documents: Incorporation certificate, partnership deed, or memorandum of association.
- Proof of Ownership/Lease of Premises: Valid rental agreement or ownership documents.
- Technical Staff Details: Qualification and experience certificates of technical personnel involved in manufacturing.
- Fire and Pollution NOCs: Approvals from local fire department and pollution control board.
- Device Master File (DMF): Comprehensive documentation on device design, manufacturing, and specifications. Our Device Master File guide can assist in preparation.
- Plant Master File (PMF): Details of manufacturing facility and equipment; refer to our Plant Master File Guide.
- Essential Principles Checklist: Compliance with CDSCO essential principles for medical devices.
- Risk Management File: Documented risk assessment and mitigation strategies, aligned with the Risk Management Guide.
- Test Reports: From CDSCO-approved testing labs confirming device safety and performance.
- Labels and Instructions for Use (IFU): Samples of packaging and user manuals.
- Quality Management System (QMS) Documents: ISO 13485 certification and internal SOPs.
Ensure all documents are prepared in line with CDSCO standards to avoid delays.
Import License Process (MD15) for Ophthalmic Head Reflector
Since the device is classified as Class A and is intended for manufacturing within India, the import license process (MD15) is generally not applicable for domestic manufacturers. However, if you plan to import this device:
- Apply for MD15 license through the CDSCO Central Licensing Authority.
- Submit relevant documents such as Manufacturing License of the exporting country, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device Master File, and Plant Master File.
- The process typically takes 5-6 months.
For more details on import licensing, visit our Import License Guide.
Timeline and Processing Duration for MD5 License
- Test License (MD13) Application: 1.5 to 2 months
- Product Testing: 1 to 1.5 months (varies by laboratory workload)
- Documentation Preparation: Concurrent with testing, 1 month recommended
- Submission and Audit: Audit scheduling and execution take approximately 1 month
- Query Resolution and License Grant: 2 to 3 weeks
Total estimated duration: Approximately 3 to 4 months from start to finish.
Timely preparation and prompt response to queries can significantly reduce delays.
Government Fees and Costs for Ophthalmic Head Reflector MD5 License
- Application Fee: Rs 5,000 per application
- Product Fee: Rs 500 per product
- Testing Charges: Vary depending on the approved laboratory (typically Rs 50,000 to Rs 1,00,000)
- Audit Fees: Usually covered by the notified body; costs vary but typically Rs 30,000 to Rs 50,000
Budgeting for these costs upfront ensures smoother financial planning.
Common Challenges and Solutions
Challenge 1: Delays in Product Testing
- Solution: Engage with testing labs early and submit samples promptly. Maintain clear communication.
Challenge 2: Incomplete Documentation
- Solution: Use checklists and expert consultation to ensure all documents meet CDSCO requirements.
Challenge 3: Audit Non-compliance
- Solution: Perform internal audits and mock inspections beforehand. Ensure QMS and manufacturing practices align with CDSCO norms.
Challenge 4: Query Resolution Delays
- Solution: Assign dedicated personnel to respond rapidly to CDSCO or notified body queries.
Expert Consultation and Support
Navigating the CDSCO licensing landscape can be complex. Our regulatory consultancy, with over 25 years of experience and 500+ successful client engagements, offers tailored support including:
- Detailed gap analysis and regulatory strategy
- Document preparation and review
- End-to-end application management on the CDSCO MD Online Portal
- Coordination with notified bodies and testing labs
- Training for technical and regulatory staff
Partnering with experts can save time, reduce costs, and ensure compliance.
Getting Started with Your CDSCO License Application for Ophthalmic Head Reflector
Assess Your Device Classification: Confirm your device is Class A and review the Medical Device Classification.
Prepare Technical Documentation: Develop your Device Master File and Plant Master File with guidance from our expert resources.
Apply for Test License (MD13): Submit your application via the CDSCO MD Online Portal.
Engage Approved Testing Laboratories: Plan and schedule product testing early.
Compile Required Documents: Use our checklists to ensure completeness.
Submit MD5 License Application (Form MD3): After successful testing, apply for the manufacturing license.
Prepare for Audit: Coordinate with a notified body from the official list for your site audit.
Respond to Queries Promptly: Maintain organized communication channels.
Receive License and Commence Manufacturing: Post license grant, ensure ongoing compliance with CDSCO regulations.
Starting with a clear, structured approach and expert assistance can make your journey to CDSCO approval for the ophthalmic head reflector smooth and successful. Reach out to us for a personalized consultation and unlock your pathway to the Indian medical device market.