CDSCO License for Ophthalmoscope
Medical Device Information
Intended Use
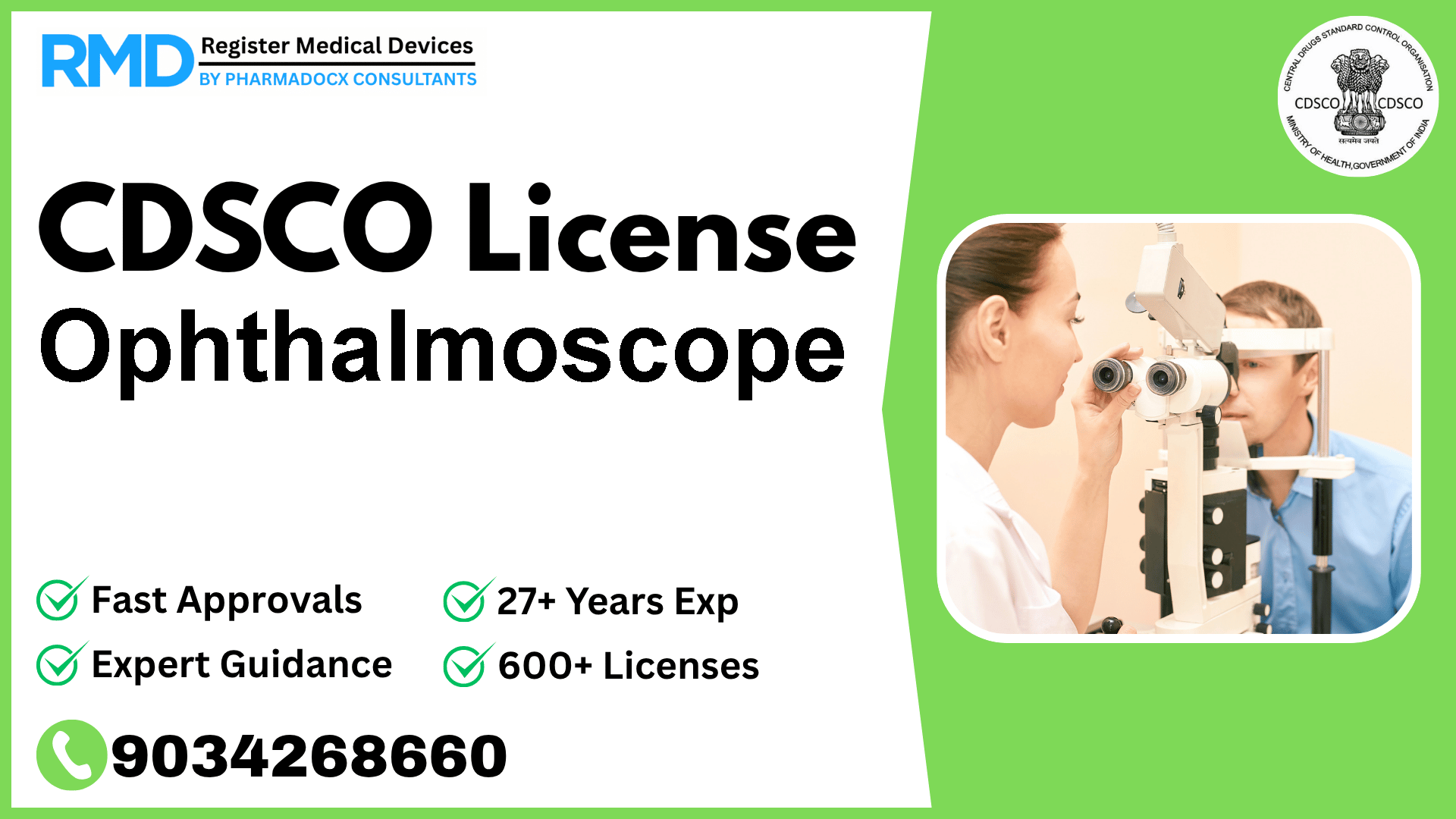
An ophthalmic instrument designed to examine the interior of the eye allowing the examiner to clearly see the details of the retina and other structures/media.
Comprehensive Guide to CDSCO Licensing for Ophthalmoscopes (Class B Medical Devices)
Ophthalmoscopes are essential diagnostic instruments in ophthalmology allowing detailed examination of the eye's interior, including the retina and other ocular structures. Given their critical role, these devices are classified as Class B under CDSCO regulations, denoting low to moderate risk. Navigating the CDSCO licensing framework effectively is crucial for manufacturers and importers aiming to market ophthalmoscopes in India.
With over 25 years of regulatory consulting experience and successful support to 500+ companies, we provide an authoritative, step-by-step overview of the licensing process, practical tips, timelines, and cost details specific to your device.
CDSCO Regulatory Framework for Ophthalmoscopes
Ophthalmoscopes fall under the ophthalmology category and are regulated per the notification Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021. This notification classifies the device as Class B and mandates compliance with the Medical Devices Rules, 2017.
The licensing authority for Class B devices is the State Licensing Authority, and the applicable manufacturing license is the MD5 license. Importers must obtain the MD15 import license from the Central Licensing Authority.
Manufacturers and importers must also ensure their devices conform to the Essential Principles of Safety and Performance, maintain a valid Quality Management System (QMS) such as ISO 13485:2016, and provide comprehensive technical documentation.
Risk Classification and License Requirements for Ophthalmoscopes
- Risk Class: B (Low to moderate risk)
- License Type for Manufacturing: MD5 License (Form MD3)
- License Authority: State Licensing Authority
- Application Portal: CDSCO MD Online Portal
The MD5 license process involves initial product testing, followed by an audit conducted by a notified body. Ophthalmoscopes, being Class B devices, require detailed documentation including Device Master File, Plant Master File, and Risk Management File.
Manufacturing License Process (MD5) for Ophthalmoscopes
The MD5 license process typically spans 3 to 4 months. Here’s a practical breakdown:
Test License (Form MD13): Obtain a test license which takes approximately 1.5 to 2 months. This allows you to manufacture the device for testing purposes.
Product Testing: Submit samples of the ophthalmoscope to CDSCO-approved laboratories. Refer to the list of testing laboratories for accredited options.
Document Preparation: Prepare and finalize all required documents including Device Master File (DMF), Plant Master File (PMF), Essential Principles Checklist, Risk Management File, and Quality Management System documents.
Application Submission: Submit the MD5 license application (Form MD3) through the CDSCO MD Online Portal.
Audit by Notified Body: A notified body from the CDSCO notified bodies list will audit your manufacturing site and QMS.
Query Resolution: Address any queries raised by the licensing authority or notified body promptly.
License Grant: Upon successful audit and document verification, the MD5 license (Form MD5) will be granted.
For a detailed guide on MD5 licensing specific to Class A and B devices, visit our MD5 License Guide.
Manufacturing License Documents Required
To ensure smooth processing, prepare the following:
- Company Constitution and Registration Documents
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing design, specifications, and manufacturing process (Device Master File Guide)
- Plant Master File (PMF) detailing infrastructure and quality systems (Plant Master File Guide)
- Essential Principles Compliance Checklist
- Risk Management File demonstrating hazard analysis and mitigation (Risk Management)
- Test Reports from CDSCO-approved labs
- Labels and Instructions for Use (IFU)
- QMS Documentation, including ISO 13485 certificates
Attention to detail in document preparation significantly reduces the risk of delays.
Import License Process (MD15) for Ophthalmoscopes
Importers looking to bring ophthalmoscopes into India must apply for the MD15 license through the Central Licensing Authority. The process takes approximately 5 to 6 months.
Key steps include:
Preparing all requisite documents such as the manufacturing license from the country of origin, Free Sale Certificate, CE Certificate, ISO 13485:2016, Device Master File, Plant Master File, Wholesale License, and Company Constitution.
Submitting the application on Form MD14 through the CDSCO MD Online Portal.
Responding to any department queries promptly.
License issuance on Form MD15.
For comprehensive steps, check our Import License Guide.
Import License Documents Required
- Valid Manufacturing License from the Country of Origin
- Free Sale Certificate issued by the Regulatory Authority of the Country of Origin
- ISO 13485:2016 Certificate
- CE Certificate or Equivalent
- Device Master File and Plant Master File
- Wholesale License issued by the State Authority
- Company Constitution and Incorporation Documents
Ensuring completeness and accuracy of these documents accelerates the licensing process.
Timeline and Processing Duration
| Process Step | Approximate Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 1 - 1.5 months |
| Document Preparation | Concurrent with Testing |
| MD5 License Application | 1 month |
| Audit and Query Resolution | 1 month |
| Total for MD5 | 3 - 4 months |
For import licenses (MD15), the complete process typically takes 5 to 6 months.
Government Fees and Costs
MD5 Manufacturing License:
- Application Fee: Rs 5,000
- Product Fee: Rs 500 per product (per device type)
MD15 Import License:
- Site Fee (Class B Device): USD 2,000 per site
- Product Fee: USD 1,000 per product
Additional costs include testing fees charged by approved laboratories and audit fees payable to notified bodies.
Common Challenges and Solutions
Delayed Test Reports: Testing laboratories often have backlogs. To mitigate this, pre-book testing slots and maintain clear communication with labs.
Incomplete Documentation: Many applicants submit insufficient or inconsistent documents. Utilize detailed checklists and consider expert review prior to submission.
Audit Non-Compliance: Notified body audits can raise unexpected observations. Prepare your staff thoroughly and maintain up-to-date QMS practices.
Query Resolution Delays: Respond promptly and comprehensively to CDSCO queries to avoid prolonged processing times.
Expert Consultation and Support
Given the complexities involved, engaging with experienced regulatory consultants can significantly streamline your licensing journey. Our team has successfully guided over 500 companies through CDSCO licensing for ophthalmic devices, ensuring compliance, timely approvals, and cost-effective strategies.
We assist with:
- Gap analysis and documentation preparation
- Coordinating with notified bodies and testing labs
- Application submission and query management
- Post-approval compliance and renewals
Getting Started with Your CDSCO License Application for Ophthalmoscopes
Assess Device Classification: Confirm your ophthalmoscope is Class B under the latest CDSCO notification.
Initiate Test License Application: Apply for the test license (Form MD13) on the CDSCO MD Online Portal.
Prepare Comprehensive Documentation: Begin compiling your Device Master File, Plant Master File, Risk Management File, and other essential documents.
Engage with Testing Labs: Schedule product testing with CDSCO-approved labs early.
Plan for Audit: Select a notified body from the CDSCO notified bodies list and prepare your facility accordingly.
Submit MD5 License Application: Following successful testing and documentation, submit your manufacturing license application (Form MD3).
Manage Queries Promptly: Stay responsive to ensure efficient approval.
Our expert team is ready to support you at every step. Contact us to leverage our 25+ years of CDSCO licensing expertise and navigate your ophthalmoscope’s market entry seamlessly.
By following this detailed roadmap and leveraging professional support, manufacturers and importers can confidently secure CDSCO licensing for ophthalmoscopes, ensuring compliance and timely access to the Indian ophthalmology market.