CDSCO License for Optical pachymeter
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
An ophthalmic, device that uses optics to measure the thickness of the cornea.
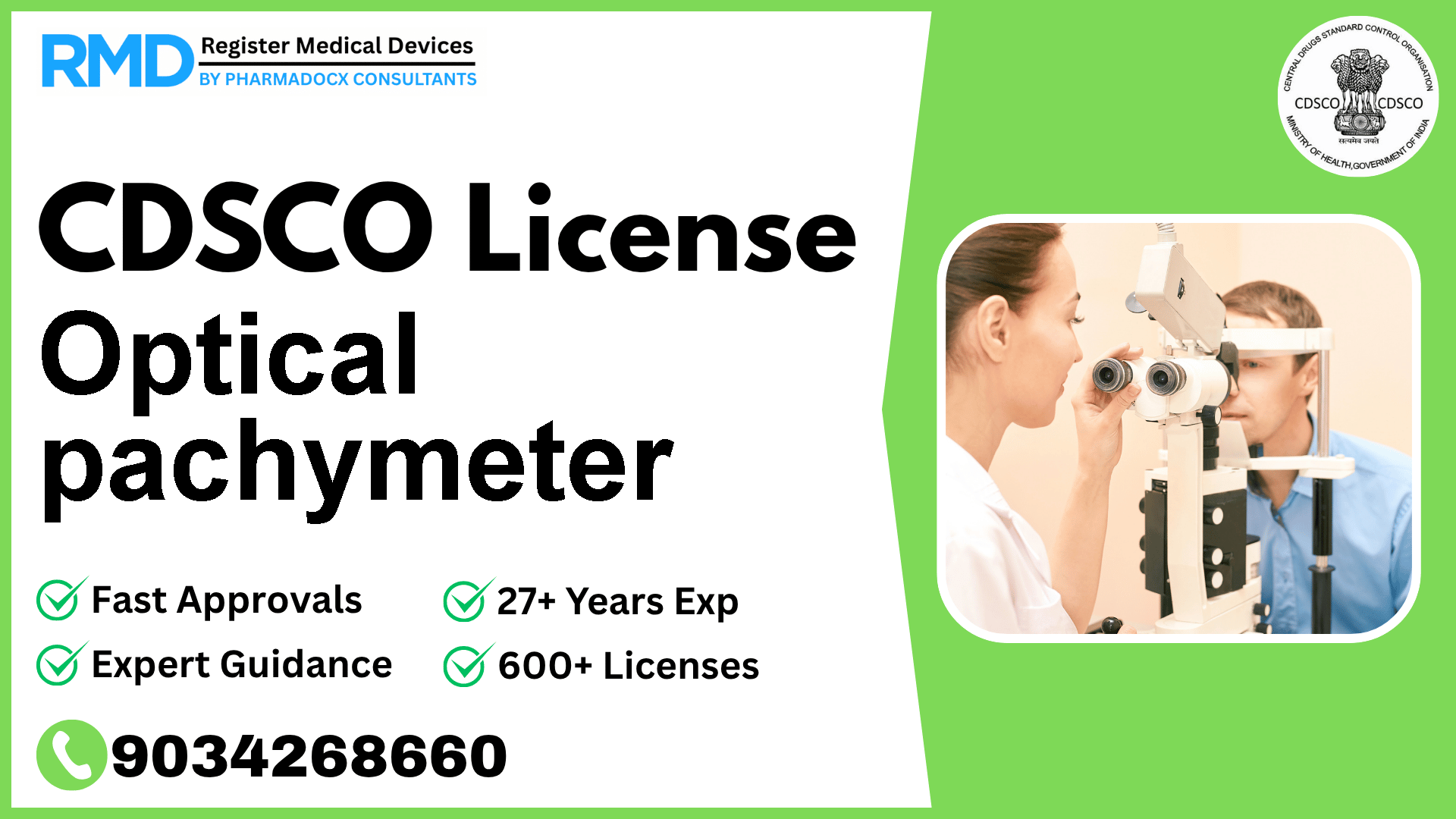
Comprehensive Guide to CDSCO Licensing for Optical Pachymeter (Class A Medical Device)
Optical pachymeters are critical ophthalmic instruments used to measure corneal thickness with precision, aiding in glaucoma management and refractive surgery planning. Given their direct impact on patient eye health, regulatory compliance with CDSCO (Central Drugs Standard Control Organization) is mandatory for manufacturers and importers seeking to market this Class A medical device in India. With over 25 years of regulatory consulting experience and successful licensing of 500+ companies, we provide a detailed roadmap to help you navigate the CDSCO approval process efficiently.
CDSCO Regulatory Framework for Optical Pachymeter
Medical devices in India are regulated under the Medical Device Rules (MDR) 2017, notified via Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021, which classifies the optical pachymeter as a Class A device. This classification denotes a low-risk device category, governed primarily by State Licensing Authorities.
The CDSCO framework requires compliance with essential principles, quality management systems, and proper documentation, along with product testing and audits before granting manufacturing or import licenses.
Risk Classification and License Requirements for Optical Pachymeter
- Device Name: Optical Pachymeter
- Risk Class: A (Low Risk)
- Intended Use: Ophthalmic device for measuring corneal thickness using optics
- Regulatory Notification: Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021
Class A devices require an MD5 Manufacturing License (Application Form MD3) issued by the State Licensing Authority. For importers, an MD15 Import License issued by the Central Licensing Authority is mandatory.
Manufacturing License Process for Optical Pachymeter (MD5 License)
The manufacturing license for Class A devices involves a multi-step process:
- Apply for a Test License (Form MD13): Initial step to legally manufacture the device for testing. Processing takes approximately 1.5 to 2 months.
- Product Testing: Conduct mandatory testing at Government-approved labs listed on the CDSCO Testing Laboratories portal.
- Document Preparation: Compile comprehensive technical and quality documentation.
- Application Submission: File Form MD3 for the MD5 license through the CDSCO MD Online Portal.
- Audit by Notified Body: Engage a notified body from the List of Notified Bodies for a facility audit.
- Query Resolution: Respond to any departmental or audit queries promptly.
- Grant of License: Receive the MD5 Manufacturing License (Form MD5).
Manufacturing License Documents Required for Optical Pachymeter
For a smooth application process, ensure all documents are complete and accurate:
- Company Constitution/Registration Certificate
- Proof of Ownership or Lease of Manufacturing Premises
- Documents of Qualified Technical Staff (e.g., Biomedical Engineers, Quality Managers)
- Fire NOC and Pollution Control NOC
- Device Master File (DMF): Detailed device design, specifications, and manufacturing process (Device Master File Guide)
- Plant Master File (PMF): Information about manufacturing facilities and quality systems (Plant Master File Guide)
- Essential Principles Checklist confirming compliance with MDR 2017
- Risk Management File following ISO 14971 principles (Risk Management Guide)
- Test Reports from approved labs
- Device Labels and Instructions for Use (IFU)
- Quality Management System Documents (ISO 13485:2016 certification recommended)
Import License Process for Optical Pachymeter (MD15 License)
If you intend to import optical pachymeters into India, the following process applies:
- Document Preparation: Gather all required documents listed below.
- Application Submission: Submit Form MD14 via the CDSCO MD Online Portal.
- Query Resolution: Address any queries from the Central Licensing Authority.
- Grant of License: Obtain MD15 Import License.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Quality Management System Certificate
- CE Certificate or equivalent international certification
- Device Master File and Plant Master File
- Wholesale Drug License in India
- Company Constitution and Registration Documents
Timeline and Processing Duration
| Process Step | Duration (Approximate) |
|---|---|
| Test License (MD13) | 1.5 to 2 months |
| Product Testing | 1 to 1.5 months |
| Application Preparation | 2 to 3 weeks |
| Application Review & Audit | 1 to 1.5 months |
| Query Resolution | 2 to 4 weeks |
| Total Time for MD5 License | 3 to 4 months |
For import licenses (MD15), expect 5 to 6 months due to centralized processing.
Government Fees and Costs
- MD5 Manufacturing License:
- Application Fee: INR 5,000 per application
- Per Product Fee: INR 500
- Test License (MD13): Included in the process, no separate fee in most states
Additional costs include notified body audit fees (variable, typically INR 50,000 - 1,00,000), government testing fees, and consultancy charges if applicable.
Common Challenges and Solutions
- Incomplete Documentation: Missing or inconsistent files delay approval. Use detailed checklists and pre-audit reviews.
- Delayed Testing: Prioritize scheduling with government-approved labs early.
- Audit Non-compliance: Prepare your quality management system to meet ISO 13485 standards rigorously.
- Query Management: Respond promptly and thoroughly to departmental queries to avoid extended delays.
Expert Consultation and Support
Our extensive experience with over 500 successful CDSCO licenses enables us to anticipate regulatory hurdles and streamline your application. We offer end-to-end support including:
- Gap analysis of your current compliance status
- Preparation of Device and Plant Master Files
- Coordination with notified bodies and testing labs
- Application drafting and submission on the CDSCO MD Online Portal
- Post-submission query handling
Getting Started with Your CDSCO License Application for Optical Pachymeter
- Assess Your Device Classification: Confirm Optical Pachymeter as Class A under MDR 2017 and your intended operation (manufacturing/import).
- Initiate Test License Application: Begin with Form MD13 for manufacturing test license if applicable.
- Plan Your Testing Strategy: Contact CDSCO-approved labs early to schedule mandatory product testing.
- Gather Documentation: Compile your Device Master File, Plant Master File, and other regulatory documents.
- Select a Notified Body: Choose from the CDSCO notified bodies for necessary audits.
- Submit Your Application: Use the CDSCO MD Online Portal for all filings.
- Prepare for Audit and Queries: Ensure compliance readiness and assign a dedicated team for timely responses.
By following these actionable steps and leveraging expert guidance, manufacturers and importers of optical pachymeters can successfully navigate the CDSCO regulatory landscape and bring their ophthalmic devices to the Indian market efficiently and compliantly.