CDSCO License for Plates, clipersScrews
Medical Device Information
Intended Use
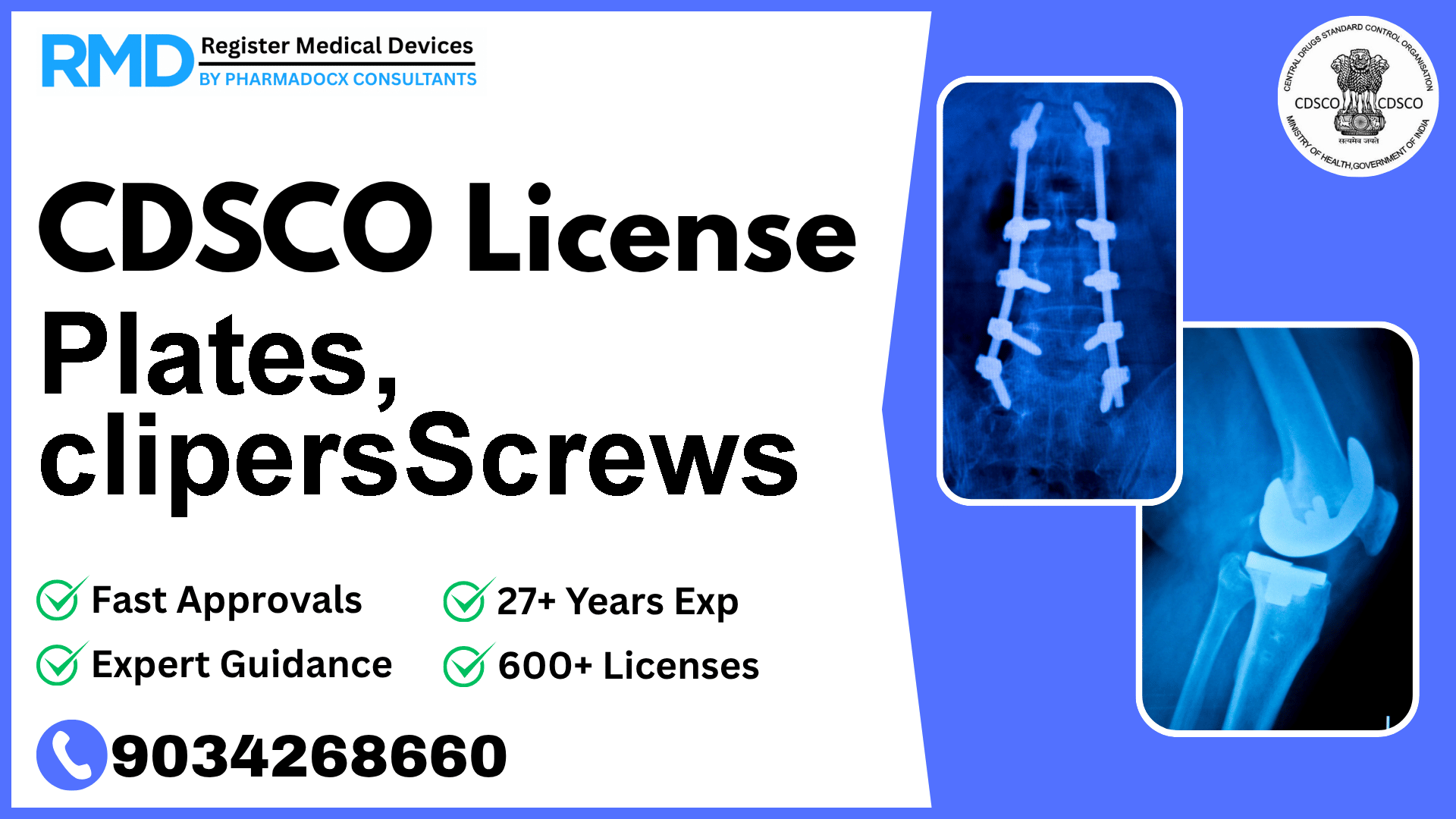
C rigid, limb brace, lumbar, lumbo- sacral, rib fracture, sacroiliac, thoracic oethosis.
Comprehensive Guide to CDSCO Licensing for Plates, Clips, and Screws (Class C Orthopaedic Implants)
Navigating the regulatory landscape for medical devices in India can be complex, especially for Class C devices like Plates, Clips, and Screws used in orthopaedic applications. With over 25 years of experience assisting more than 500 companies, we provide you with a detailed, step-by-step roadmap to successfully obtain your CDSCO license, ensuring compliance and market access for your devices.
Understanding Your Device and Its Regulatory Importance
Plates, Clips, and Screws are critical orthopaedic implants designed to support rigid fixation in limbs, lumbar, lumbo-sacral, rib fractures, sacroiliac, and thoracic orthoses. Given their invasive nature and significant impact on patient health, these devices fall under Class C risk category as per CDSCO classification, requiring stringent regulatory oversight to guarantee safety and performance.
The official notification governing these devices is Notification 29/Misc/3/2017-DC (292), dated 06.06.2018, ensuring they meet the essential principles laid down by CDSCO.
CDSCO Regulatory Framework for Plates, Clips, and Screws
Class C devices like orthopaedic implants are regulated centrally by CDSCO, involving a comprehensive evaluation process that includes:
- Product testing by government-approved laboratories
- Submission of detailed technical and quality documentation
- On-site audits by CDSCO inspectors
This rigorous framework assures stakeholders of the device’s safety and efficacy.
Risk Classification and License Requirements for Class C Devices
For your device category, the license required is the MD9 Manufacturing License, granted by the Central Licensing Authority. This process is detailed, reflecting the higher risk class and critical nature of orthopaedic implants.
You can review the medical device classification criteria to confirm your device’s category.
Manufacturing License Process (MD9) for Plates, Clips, and Screws
The MD9 license process is comprehensive and includes the following key steps:
- Test License (Form MD13): Obtain a test license first, allowing product testing. This usually takes 1.5 to 2 months.
- Product Testing: Get your devices tested at CDSCO-approved labs to confirm compliance. Refer to the list of testing laboratories.
- Document Preparation: Prepare exhaustive technical documentation including Device Master File, Plant Master File, Risk Management File, and Quality Management System (QMS) documents.
- Application Submission (Form MD7): Submit the MD9 application through the CDSCO MD Online Portal.
- On-site Audit: CDSCO inspectors conduct audits of your manufacturing facility.
- Query Resolution: Address any queries raised by the department or inspectors promptly.
- License Grant (Form MD9): Upon satisfactory audit and document review, CDSCO issues the manufacturing license.
Manufacturing License Documents Required
For Class C orthopaedic implants like Plates, Clips, and Screws, you must prepare and submit the following documents:
- Company Constitution (Incorporation Certificate, Memorandum & Articles of Association)
- Proof of Ownership or Lease of Manufacturing Premises
- Qualification and Experience Details of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF): Detailed device information and specifications (See our Device Master File guide)
- Plant Master File (PMF): Manufacturing process, equipment details, and quality controls (Learn how to create a Plant Master File)
- Essential Principles Compliance Checklist
- Risk Management File highlighting hazard analysis and mitigation strategies (Detailed Risk Management guide)
- Test Reports from CDSCO-approved laboratories
- Device Labels and Instructions For Use (IFU)
- Quality Management System documents (ISO 13485:2016 certification preferred)
Import License Process (MD15) for Orthopaedic Implants
If you are importing Plates, Clips, and Screws, an MD15 Import License is mandatory. Key points include:
- Application submission via Form MD14 on the CDSCO MD Online Portal
- No test license required, but documentation must demonstrate compliance
- Required documents include:
- Valid Manufacturing License from country of origin
- Free Sale Certificate
- ISO 13485:2016 and CE Certificates
- Device and Plant Master Files
- Wholesale Drug License
- Company Constitution
The import license process generally takes around 5-6 months.
Timeline and Processing Duration
| License Type | Process Steps | Estimated Duration |
|---|---|---|
| MD9 Manufacturing | Test license, testing, audit | 4-5 months total |
| MD15 Import License | Document review, queries | 5-6 months total |
The test license (MD13) alone takes 1.5-2 months, followed by testing and audit phases. We recommend initiating early to accommodate any unforeseen delays.
Government Fees and Costs
MD9 Manufacturing License:
- Application Fee: ₹50,000 per application
- Product Fee: ₹1,000 per product
MD15 Import License: Fees vary by class, with Class C devices attracting ₹3,000 per site and ₹1,500 per product
Note: Fees are payable online through the CDSCO portal and are subject to change; always verify current fees before application.
Common Challenges and Practical Solutions
Challenge 1: Delays in Testing and Audit Scheduling
- Solution: Engage with notified testing laboratories early and schedule audits proactively. Refer to the list of notified bodies for audit requirements.
Challenge 2: Incomplete or Non-compliant Documentation
- Solution: Ensure thorough preparation of Device and Plant Master Files using established templates and expert consultation.
Challenge 3: Resolving Regulatory Queries
- Solution: Maintain prompt and clear communication with CDSCO officials. Prepare comprehensive responses backed by technical evidence.
Expert Consultation and Support
We have successfully guided over 500 manufacturers and importers through the CDSCO licensing maze for Class C devices, including orthopaedic implants like Plates, Clips, and Screws. Our services include:
- Customized document preparation and review
- Liaison with notified bodies and testing labs
- Complete application filing and follow-up
Our deep regulatory knowledge ensures your application is robust, reducing rejection risk and accelerating approval.
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm Class C status using our classification guide.
- Prepare Test License Application (Form MD13): Initiate testing preparations early.
- Engage with a CDSCO-Approved Testing Laboratory: Start sample testing as soon as test license is granted.
- Compile Required Documentation: Use expert templates and consult our Device and Plant Master File guides.
- Submit Your Application via the CDSCO MD Online Portal: Ensure all fees are paid and forms correctly completed.
- Prepare for Audit: Organize your facility and QMS documentation for CDSCO inspection.
- Address Queries Quickly: Respond to any feedback from CDSCO promptly to avoid delays.
Embarking on this regulatory journey with professional backing maximizes your chances of timely approval and successful market entry. Contact us today to leverage our 25+ years of CDSCO licensing expertise for your orthopaedic implant manufacturing or import business.