CDSCO License for Powered neutron therapy table
Medical Device Information
Intended Use
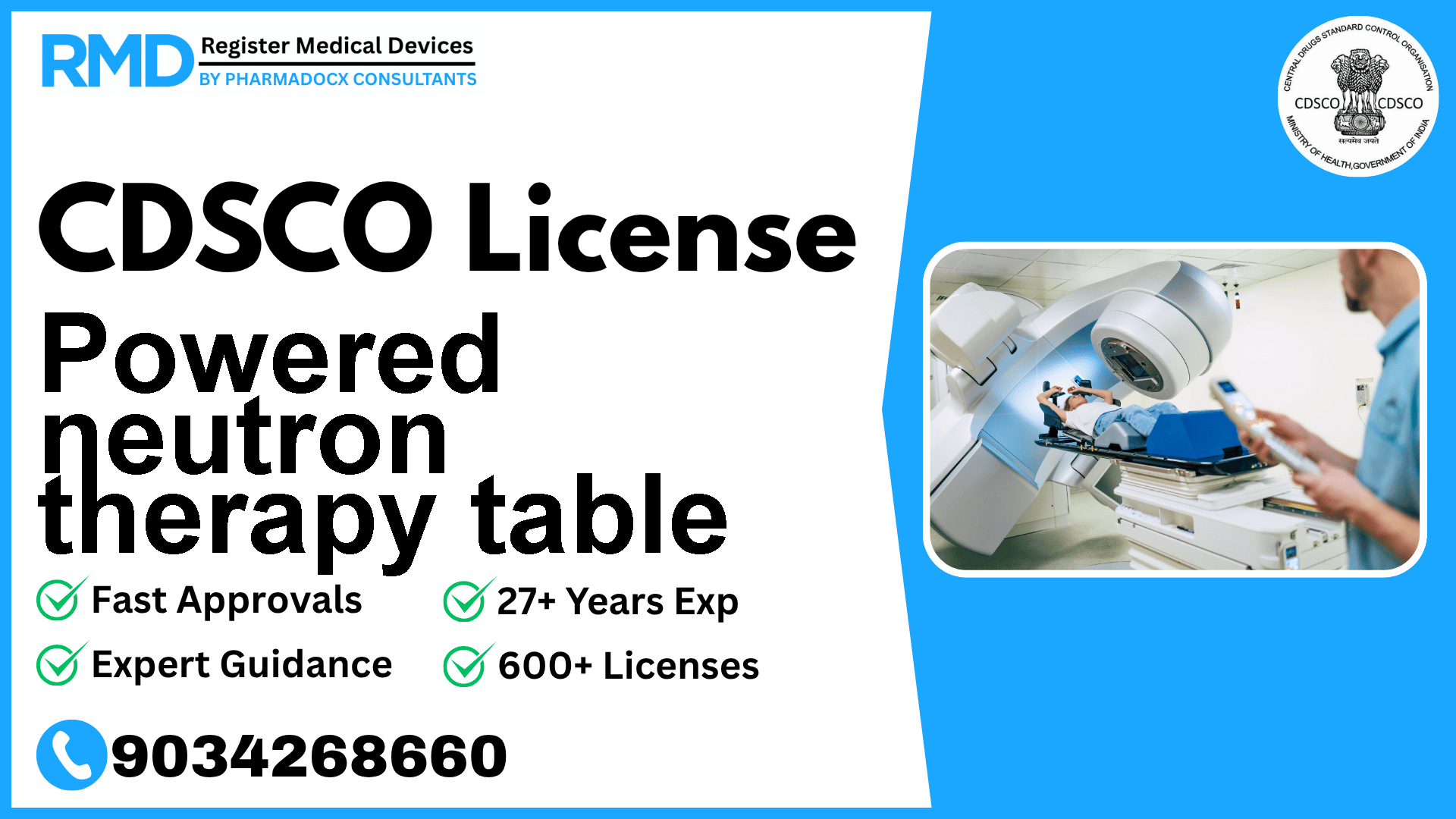
A programmable bed for radiotherapy designed to adjust the patient's posture and immobilize the patient for treatment that uses neutron rays that are generated from the nuclear reactor, etc.
Introduction to Powered Neutron Therapy Table and CDSCO Licensing
The powered neutron therapy table is a specialized radiotherapy device designed to precisely position and immobilize patients during neutron ray treatments, which originate from nuclear reactors. Its programmable features ensure accurate patient posture adjustment, crucial for effective radiotherapy outcomes. Given its critical role in cancer treatment and associated patient safety concerns, it falls under Risk Class B within the Indian regulatory framework.
Navigating the Central Drugs Standard Control Organization (CDSCO) licensing process is essential for manufacturers or importers aiming to market powered neutron therapy tables in India. With over 25 years of regulatory consulting experience and having assisted more than 500 companies, we provide you with an in-depth, actionable roadmap tailored for this device.
CDSCO Regulatory Framework for Powered Neutron Therapy Table
Powered neutron therapy tables are categorized under radiotherapy medical devices and regulated under the Medical Device Rules (MDR) 2017, amended from time to time. The CDSCO acts as the central authority governing the licensing process, quality compliance, and post-market surveillance.
Given the device's classification as Risk Class B, it is subject to a State Licensing Authority's jurisdiction. This classification mandates specific conformity assessments, testing, and documentation to ensure safety and efficacy.
Risk Classification and License Requirements
According to the Medical Device Rules, powered neutron therapy tables fall under Class B due to their moderate risk profile involving patient immobilization and interaction with neutron radiation.
The applicable manufacturing license is the MD5 license (Application Form MD3) issued by the State Licensing Authority. For importers, the MD15 import license applies if the product is sourced internationally.
You can verify classification details on the Medical Device Classification guide.
Manufacturing License Process (MD5) for Class B Devices
The MD5 license process involves several critical steps:
Test License Acquisition (Form MD13): Before full manufacturing license application, manufacturers must secure a test license. This allows for product testing and validation.
Product Testing: Testing must be performed at government-approved laboratories listed on the CDSCO Testing Laboratories portal. Testing focuses on safety, performance, and compliance with Essential Principles.
Document Preparation: Compile comprehensive documentation including Device Master File, Plant Master File, Risk Management File, Quality Management System (QMS) documents, and other regulatory paperwork.
License Application (Form MD3): Submit the manufacturing license application via the CDSCO MD Online Portal along with the required fees.
Audit by Notified Body: A notified body listed here conducts an on-site audit of manufacturing premises and quality systems.
Queries Resolution: Address any queries raised by the State Licensing Authority or the notified body promptly.
Grant of MD5 License: Upon successful audit and documentation review, the license is granted.
Manufacturing License Documents Required for Powered Neutron Therapy Table
The documentation is extensive and must be meticulously prepared:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Qualification and Experience Certificates of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF): Detailed device design, specifications, and manufacturing process (Device Master File guide)
- Plant Master File (PMF): Facility layout, equipment, and quality control procedures (Plant Master File guide)
- Essential Principles Checklist demonstrating compliance with Indian MDR
- Risk Management File documenting hazard analysis and mitigation strategies (Risk Management guide)
- Test Reports from accredited laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System documentation compliant with ISO 13485:2016
Import License Process (MD15) for Powered Neutron Therapy Table
If importing the powered neutron therapy table, an MD15 license is mandatory, granted by the Central Licensing Authority. The process is as follows:
Document Preparation: Collect all necessary documents, including the manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device and Plant Master Files, and wholesale license.
Application Submission (Form MD14): File the import application via the CDSCO MD Online Portal.
Queries Resolution: Respond to any departmental queries.
Grant of MD15 License: Upon satisfactory review, the import license is issued.
Import License Documents Required
- Valid Manufacturing License from Exporting Country
- Free Sale Certificate
- ISO 13485:2016 Certification
- CE Certificate or equivalent
- Device Master File and Plant Master File
- Wholesale Drug License
- Company Constitution and Incorporation Documents
Timeline and Processing Duration
For the powered neutron therapy table (Class B), expect the following:
- Test License (MD13): Approximately 1.5 to 2 months
- Product Testing: 1 to 1.5 months depending on lab workload
- Audit and Application Processing: 1 to 1.5 months
- Total Duration: Around 3 to 4 months from test license to final MD5 license grant
Import license (MD15) typically takes 5 to 6 months due to document verification and regulatory scrutiny.
Government Fees and Costs
- MD5 License Application Fee: ₹5,000 per application plus ₹500 per product
- Test License Fee: Included in application fees but may vary by state
- Audit Charges: Paid to notified bodies separately, often ranging ₹50,000 to ₹1,00,000 depending on audit scope
Additional costs include testing fees at government-approved laboratories, which typically range from ₹50,000 to ₹1,00,000 depending on device complexity.
Common Challenges and Solutions
- Delayed Test Reports: Coordinate early with testing labs to avoid bottlenecks.
- Incomplete Documentation: Use checklists aligned with CDSCO requirements. Refer to our detailed Device Master File guide to ensure completeness.
- Audit Non-Conformities: Conduct internal pre-audit assessments to identify gaps.
- Query Resolution Delays: Assign dedicated regulatory personnel to address queries promptly.
Expert Consultation and Support
Navigating CDSCO licensing for specialized devices like powered neutron therapy tables can be complex. Our team, with over 25 years of experience and 500+ successful license approvals, offers:
- Comprehensive gap analysis and readiness assessment
- Documentation preparation and review
- Coordination with testing laboratories and notified bodies
- End-to-end application filing and follow-up
Getting Started with Your CDSCO License Application
Assess Device Classification & Prepare Documentation: Confirm your device risk class as B and begin compiling the Device Master File and Plant Master File.
Apply for Test License (MD13): Submit your test license application through the CDSCO MD Online Portal.
Schedule Product Testing: Engage with government-approved testing laboratories early to plan testing.
Pre-Audit Preparation: Conduct an internal audit using notified body checklists obtained from the Notified Bodies List.
Submit MD5 License Application: Once testing and audits are completed successfully, proceed with Form MD3 application.
Monitor Application: Track your application status regularly and respond promptly to queries.
By following these steps and leveraging expert guidance, manufacturers and importers can efficiently obtain CDSCO licenses to market powered neutron therapy tables in India, ensuring compliance and patient safety.
For personalized support, reach out to our regulatory consulting team to streamline your CDSCO licensing journey.