CDSCO License for Powered radionuclide brachytherapy table
Medical Device Information
Intended Use
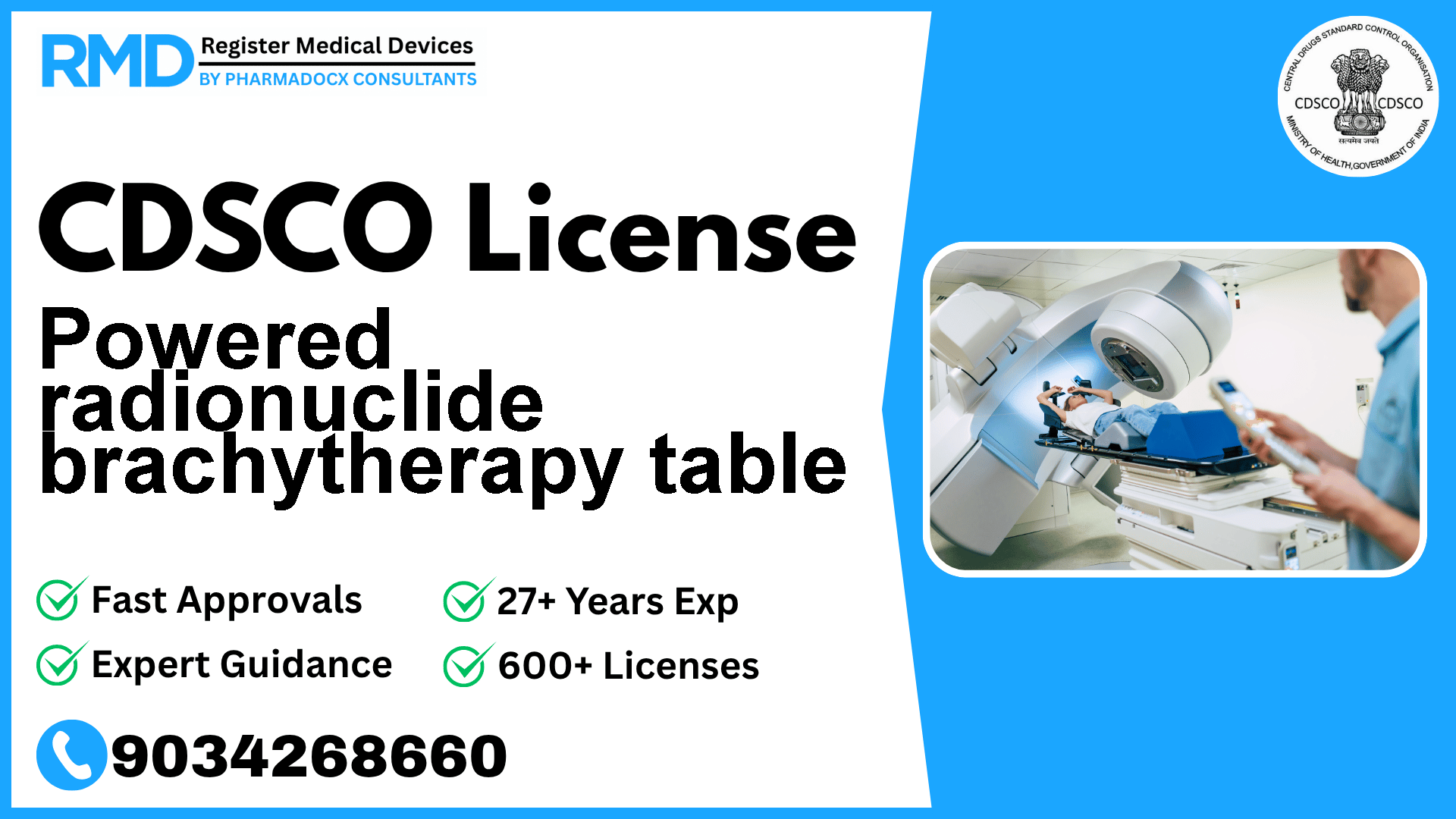
A programmable bed for radiotherapy designed to adjust the patient's posture and immobilize the patient for treatment that uses an after loading short-distance irradiation treatment apparatus that is operated manually or electrically.
Introduction to Powered Radionuclide Brachytherapy Table and Its Regulatory Importance
The Powered radionuclide brachytherapy table is a sophisticated medical device designed specifically for radiotherapy applications. It is a programmable bed that adjusts patient posture and immobilizes patients during short-distance irradiation treatments using an afterloading apparatus operated manually or electrically. Given the critical nature of radiotherapy and patient safety concerns, regulatory compliance with the Central Drugs Standard Control Organization (CDSCO) is mandatory for manufacturers and importers seeking to market this device in India.
Regulatory approval ensures that your device meets stringent safety, quality, and performance standards, protecting patients and healthcare providers while facilitating smooth market access.
CDSCO Regulatory Framework for Powered Radionuclide Brachytherapy Table
According to CDSCO’s medical device notification (File No. 29/Misc./03/2020-DC (180), dated 6.8.2021), the Powered radionuclide brachytherapy table is classified under Risk Class B for radiotherapy devices. This device falls within the scope of the Medical Device Rules (MDR) 2017, and compliance with these rules is essential.
Manufacturers intending to produce this device in India must obtain a manufacturing license, while importers must secure an import license from the CDSCO. The regulatory framework mandates adherence to quality management systems (QMS) such as ISO 13485:2016, device testing, and audit by notified bodies or CDSCO inspectors.
Risk Classification and License Requirements for Class B Devices
The Powered radionuclide brachytherapy table is classified as a Class B device due to its moderate risk profile. Under the CDSCO classification:
- Class A & B devices: Licensing authority is the State Licensing Authority.
- License type for Class B: MD5 Manufacturing License (Application Form MD3).
- Import License: MD15 (through Central Licensing Authority).
Class B devices require a comprehensive yet streamlined approval process involving a test license, product testing, audit, and documentation review.
For more on device classification, visit our detailed Medical Device Classification guide.
Manufacturing License Process (MD5 License) for Class B Devices
Obtaining an MD5 license for your Powered radionuclide brachytherapy table involves several well-defined steps:
- Test License on Form MD13: Apply first for a test license to manufacture the device for testing purposes. This is valid for 6 months and takes approximately 1.5 to 2 months for approval.
- Product Testing: Conduct product testing at CDSCO-approved laboratories to demonstrate conformity to essential principles and safety standards. Refer to the Testing Laboratories list for authorized labs.
- Document Preparation: Compile all required documents including Device Master File (DMF), Plant Master File (PMF), risk management files, test reports, and quality management system documents.
- Application Submission: Submit your manufacturing license application on Form MD3 through the CDSCO MD Online Portal.
- Audit by Notified Body: Undergo an audit by a CDSCO-recognized notified body to verify compliance. You can check the list of notified bodies for audit services.
- Query Resolution: Promptly address any queries or deficiencies raised by the CDSCO or notified body.
- Grant of License: Upon satisfactory review, the State Licensing Authority issues the MD5 manufacturing license.
The entire process typically spans 3 to 4 months, including testing and audit phases.
For a detailed stepwise procedure, refer to our comprehensive MD5 License Guide.
Manufacturing License Documents Required
To ensure smooth processing of your MD5 application, prepare the following key documents:
- Company Constitution (Incorporation Certificate, Partnership Deed, etc.)
- Proof of ownership or lease of manufacturing premises
- Details and qualifications of technical staff
- Fire Safety No Objection Certificate (NOC)
- Pollution Control Board NOC
- Device Master File (detailed design, specifications, and manufacturing process) - see our Device Master File guide
- Plant Master File (plant layout, equipment details) - refer to Plant Master File guide
- Essential Principles Checklist demonstrating compliance with CDSCO requirements
- Risk Management File per ISO 14971 standards - learn more in our Risk Management guide
- Test Reports from CDSCO-approved labs
- Product labels, packaging, and Instructions for Use (IFU)
- Quality Management System documents (ISO 13485:2016 certification preferred)
Organizing these documents thoroughly reduces back-and-forth with regulators and expedites approval.
Import License Process (MD15 License) for Powered Radionuclide Brachytherapy Table
For importers, obtaining an MD15 import license from the Central Licensing Authority is mandatory before market entry. The process includes:
- Document Preparation: Collect required documents such as valid manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016 certificate, CE Certificate (if applicable), Device Master File, Plant Master File, Wholesale License, and Company Constitution.
- Application Submission: Submit the application on Form MD14 through the CDSCO MD Online Portal.
- Evaluation & Query Resolution: CDSCO reviews documents and may raise queries.
- Granting License: Once all conditions are met, the import license on Form MD15 is granted.
The import license process usually takes 5 to 6 months, with fees based on risk class and number of products.
For a full walkthrough, see our Import License Guide.
Import License Documents Required
Key documents for MD15 license application include:
- Manufacturing License from country of origin
- Free Sale Certificate from the country of origin
- ISO 13485:2016 Quality Management System certificate
- CE Certificate or equivalent
- Device Master File & Plant Master File
- Wholesale Drug License (if applicable in India)
- Company Constitution and address proof
- Product brochures, labels, and IFU
Ensure authenticity and proper translation of foreign documents to avoid delays.
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Document Preparation | 2 – 3 weeks |
| Application Submission & Audit | 1 – 1.5 months |
| Query Resolution | 2 – 4 weeks |
| Total (MD5 License) | 3 – 4 months |
For import license (MD15), the entire process extends to 5 – 6 months.
Government Fees and Costs
For Class B devices like the Powered radionuclide brachytherapy table, the government fees applicable are:
- MD5 Manufacturing License: Rs 5,000 per application + Rs 500 per product
- Test License (MD13): Included within the manufacturing license process
Additional costs to consider:
- Product testing fees at notified labs
- Audit fees charged by notified bodies
- Consultancy fees if you engage expert support
Budgeting realistically upfront helps avoid surprises.
Common Challenges and Practical Solutions
Challenge 1: Delays in product testing due to limited slots at notified labs.
Solution: Schedule testing early; maintain good communication with testing labs listed here.
Challenge 2: Incomplete or poorly organized documentation causing multiple query rounds.
Solution: Use detailed checklists and professional document management; consider templates for DMF and PMF.
Challenge 3: Audit non-compliance due to inadequate QMS or facility preparedness.
Solution: Conduct internal audits and gap analysis before the official audit; train technical staff thoroughly.
Challenge 4: Unfamiliarity with Indian regulatory nuances leading to application errors.
Solution: Engage regulatory consultants with CDSCO experience; leverage our 25+ years of guidance for over 500 companies.
Expert Consultation and Support
Navigating CDSCO licensing for specialized devices like the Powered radionuclide brachytherapy table can be complex. With our extensive experience in regulatory consulting, we provide tailored support including:
- Gap assessments and readiness audits
- Documentation preparation and review
- Liaison with CDSCO and notified bodies
- Product testing coordination
- Training and compliance strategy
Our proven methodologies reduce approval times and increase the likelihood of first-time success.
Getting Started with Your CDSCO License Application
Begin your regulatory journey with these actionable steps:
- Assess your device classification and licensing path: Confirm Class B categorization and MD5 license applicability.
- Prepare essential documentation: Start compiling the Device Master File, Plant Master File, and risk management documents.
- Apply for the test license (Form MD13): Submit the application early via the CDSCO MD Online Portal.
- Select a notified laboratory: Book your product testing slot well in advance.
- Plan for audit readiness: Arrange your technical staff and facility in compliance with CDSCO standards.
- Engage expert consultants: Consider professional regulatory support to streamline the process.
By methodically following these steps and leveraging our expertise, manufacturers and importers can successfully obtain CDSCO approval for the Powered radionuclide brachytherapy table and confidently enter the Indian radiotherapy market.
For detailed guidance and personalized assistance, contact our regulatory experts today.