CDSCO License for Pupillograph
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A graphic recorder used for recording the response of the pupil to reflected light. It is used for ophthalmic diagnostic purposes.
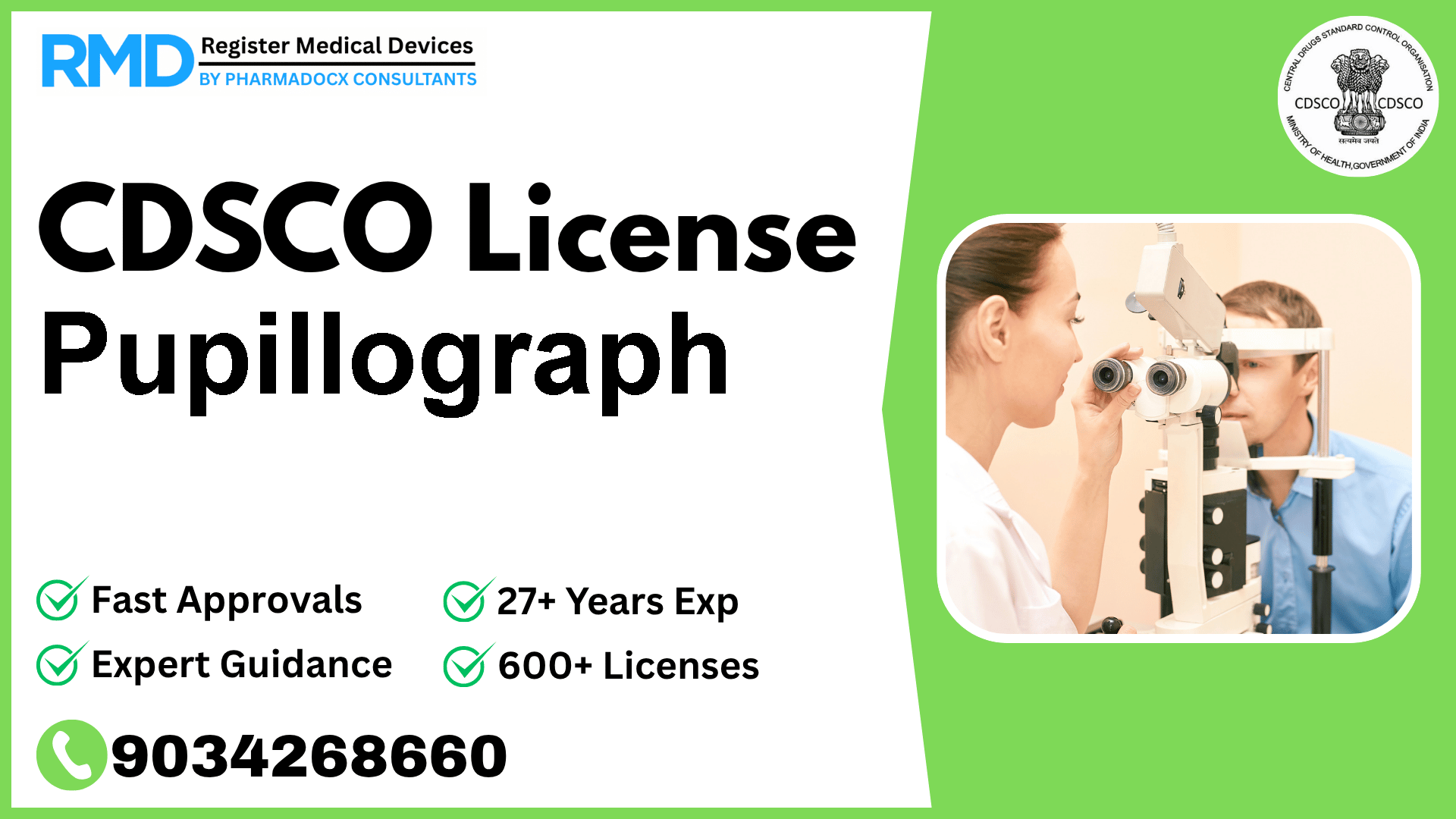
Introduction to Pupillograph and Its Regulatory Importance
The Pupillograph is an advanced ophthalmic device designed to graphically record the pupil's response to reflected light, offering critical diagnostic insights. As a Class A medical device under the Indian regulatory framework, the Pupillograph’s compliance with CDSCO (Central Drugs Standard Control Organisation) regulations is mandatory for manufacturers and importers aiming to market this device in India. Navigating the licensing process efficiently ensures timely market access and compliance with safety and quality standards.
CDSCO Regulatory Framework for Pupillograph (Class A Device)
The CDSCO classifies medical devices into four classes (A, B, C, D) based on risk, with Class A representing the lowest risk category. The Pupillograph falls under Class A as per the Medical Device Rules 2017, notified under Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021. Regulatory oversight for Class A devices is primarily through the State Licensing Authority via the MD5 license.
Risk Classification and License Requirements for Pupillograph
Being a Class A device, the Pupillograph requires an MD5 manufacturing license. This license confirms that the manufacturing processes comply with established quality and safety norms. The regulatory pathway involves initial test licensing, product testing, document submission, and audits by a notified body.
Manufacturing License Process (MD5) for Pupillograph
- Test License (Form MD13): Before full manufacturing approval, you must obtain a test license valid for 12 months. This allows sample production for testing.
- Product Testing: Samples must be tested at CDSCO-approved government laboratories. Refer to the Testing Laboratories list for authorized facilities.
- Document Preparation: Assemble all required technical and compliance documents.
- Application Submission (Form MD3): Submit the MD5 license application via the CDSCO MD Online Portal.
- Audit by Notified Body: An audit by a notified body verifies compliance with quality systems and manufacturing premises.
- Query Resolution: Address any queries raised during review or audit promptly.
- License Grant (Form MD5): Upon successful review and audit, the State Licensing Authority issues the manufacturing license.
For a detailed walkthrough, see our MD5 License Guide.
Manufacturing License Documents Required for Pupillograph
- Company Constitution (Certificate of Incorporation, Partnership Deed, etc.)
- Proof of Ownership or Lease Agreement of manufacturing premises
- Technical Staff Qualification & Experience Records
- Fire NOC and Pollution Control NOC
- Device Master File (DMF) detailing design and specifications (Device Master File Guide)
- Plant Master File outlining manufacturing processes and quality systems (Plant Master File Guide)
- Essential Principles Checklist confirming compliance with regulatory standards
- Risk Management File showing risk analysis and mitigation (Risk Management)
- Test Reports from government-approved laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) Documents such as ISO 13485 implementation and SOPs
Import License Process (MD15) for Pupillograph
For importers, an MD15 import license is required, granted by the Central Licensing Authority. Unlike manufacturing, the MD15 license does not mandate a test license, but requires comprehensive documentation including a valid manufacturing license from the country of origin, Free Sale Certificate, ISO 13485 certification, CE Certificate (if applicable), and Company Constitution.
Application is submitted through the CDSCO MD Online Portal with the Form MD14. The process typically spans 5-6 months.
For more details, consult our Import License Guide.
Import License Documents Required for Pupillograph
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certification
- CE Certificate (if marketed in Europe)
- Device Master File
- Plant Master File
- Wholesale License (if applicable)
- Company Constitution
Timeline and Processing Duration
MD5 License Process: Approximately 3-4 months
- Test License (MD13): 1.5-2 months
- Product Testing: 2-3 weeks
- Document preparation and submission: 2-3 weeks
- Audit and review: 3-4 weeks
- Query resolution and license grant: 2-3 weeks
MD15 Import License: Approximately 5-6 months
Understanding these timelines helps manufacturers and importers plan resource allocation and market entry strategies effectively.
Government Fees and Costs for Pupillograph Licensing
MD5 License:
- Application Fee: ₹5,000
- Per Product Fee: ₹500
MD15 Import License (Class A device):
- Site Fee: $1000
- Per Product Fee: $50
These fees are payable online through the CDSCO portal during application submission.
Common Challenges and Practical Solutions
- Delayed Test Reports: Coordinate early with testing labs to avoid bottlenecks. Choose from the list of CDSCO-approved testing laboratories.
- Incomplete Documentation: Use comprehensive checklists and expert consultation to ensure completeness.
- Audit Observations: Prepare the manufacturing site in advance and maintain up-to-date QMS records.
- Query Resolution Delays: Assign dedicated regulatory personnel to respond promptly.
Our experience with over 500 companies confirms that proactive planning and adherence to regulatory standards mitigate most common issues.
Expert Consultation and Support
Navigating CDSCO licensing for Class A devices like the Pupillograph requires deep understanding of regulatory nuances. Our expert team offers:
- Tailored document preparation and review
- Audit readiness support
- Liaison with CDSCO officials and notified bodies
- End-to-end application management
Partnering with experienced consultants accelerates approval timelines and ensures compliance.
Getting Started with Your CDSCO License Application for Pupillograph
- Evaluate your device classification and confirm Pupillograph as Class A.
- Register your company and create an account on the CDSCO MD Online Portal.
- Initiate your test license (MD13) application to begin sample manufacturing.
- Engage authorized testing laboratories early to schedule product testing.
- Prepare your Device Master File and Plant Master File using detailed guides.
- Compile all required documents and submit your MD5 license application (Form MD3).
- Coordinate audit schedules with notified bodies from the Notified Bodies List.
- Address queries swiftly and track application status regularly.
By following these actionable steps and leveraging experienced regulatory support, you can successfully obtain your CDSCO manufacturing or import license for the Pupillograph and bring your ophthalmic diagnostic device to the Indian market with confidence.