CDSCO License for Pupillometer
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
An ophthalmic instrument used for measuring the width or diameter of the pupil.
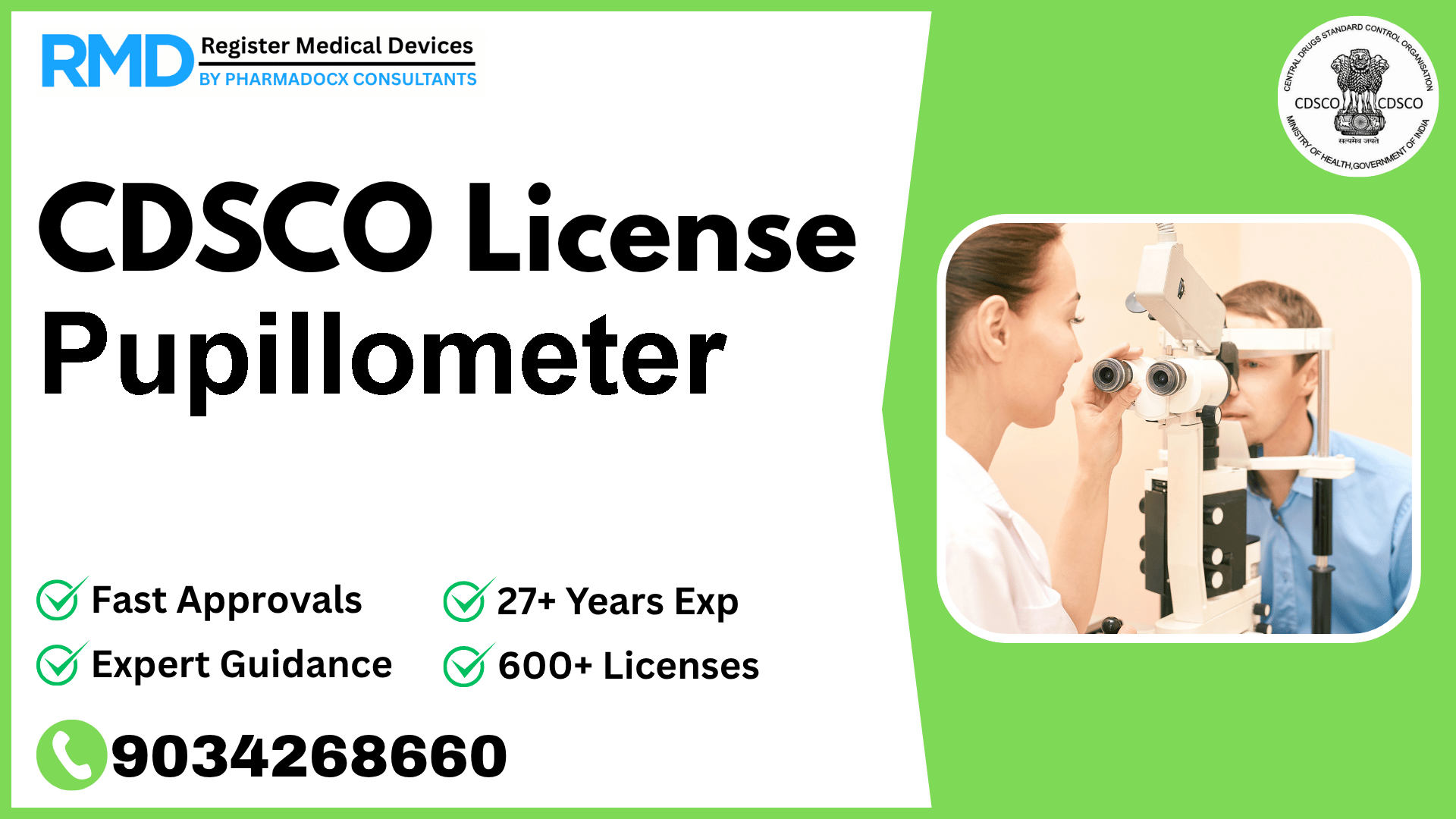
Comprehensive Guide to CDSCO Licensing for Pupillometers (Class A Medical Device)
Pupillometers, classified under Class A medical devices, are specialized ophthalmic instruments designed to measure the width or diameter of the pupil. As an essential diagnostic tool in ophthalmology, regulatory compliance with the Central Drugs Standard Control Organization (CDSCO) is mandatory before marketing or manufacturing this device in India. With over 25 years of experience assisting 500+ manufacturers and importers, we provide you with a detailed roadmap to obtain your CDSCO license swiftly and efficiently.
CDSCO Regulatory Framework for Pupillometers (Class A)
The regulatory landscape for medical devices in India is governed by the Medical Device Rules, 2017, under the Ministry of Health and Family Welfare. Pupillometers fall under Class A (low-risk) medical devices as per the notification Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021. This classification dictates the licensing pathway, documentation, and the authority responsible for granting the manufacturing license.
Risk Classification and License Requirements
- Device: Pupillometer
- Risk Class: A (Low Risk)
- Category: Ophthalmology
- License Type: MD5 (Manufacturing License for Class A & B Devices)
- Licensing Authority: State Licensing Authority
Class A devices require an MD5 license, obtained by submitting Form MD3 on the CDSCO MD Online Portal. This process also necessitates a test license (Form MD13) prior to the final manufacturing license application.
Manufacturing License Process (MD5) for Pupillometers
The manufacturing licensing process for Class A devices like Pupillometers typically takes 3 to 4 months and involves the following key steps:
Apply for Test License (Form MD13): Initially, a test license is granted for conducting product testing in CDSCO-approved laboratories. This phase takes approximately 1.5 to 2 months.
Product Testing: You must get your Pupillometer tested at government-approved labs to verify compliance with Indian standards. Refer to the list of testing laboratories authorized by CDSCO.
Document Preparation: Compile mandatory documentation including Device Master File, Plant Master File, Risk Management File, and Quality Management System (QMS) documents.
Submit Application for Manufacturing License (Form MD3): Upon successful testing, submit the MD5 license application through the CDSCO MD Online Portal.
Audit by Notified Body: An audit by a notified body is conducted to verify manufacturing compliance. You can check the list of notified bodies authorized for Class A device audits.
Resolve Queries: Address any queries raised by the licensing authority or notified body promptly.
Grant of MD5 License: After successful audit and query resolution, the license is granted on Form MD5.
Manufacturing License Documents Required for Pupillometers
Preparing a comprehensive documentation package is critical. For Pupillometers (Class A), the following documents must be submitted:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing design, specifications, and manufacturing process (Device Master File Guide)
- Plant Master File (PMF) describing manufacturing infrastructure (Plant Master File Guide)
- Essential Principles Checklist confirming compliance with Indian Medical Device Rules
- Risk Management File outlining hazard analysis and mitigation strategies (Risk Management)
- Test Reports from CDSCO-approved laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System Documents (ISO 13485:2016 certification is highly recommended)
Import License Process (MD15) for Pupillometers
If you are an importer of Pupillometers, an MD15 Import License is mandatory, issued by the Central Licensing Authority. The process typically takes 5 to 6 months and involves:
- Compiling regulatory documents including existing manufacturing license, Free Sale Certificate, ISO 13485:2016 Certificate, CE Certificate, Device Master File, and Plant Master File.
- Applying using Form MD14 on the CDSCO MD Online Portal.
- Responding to queries from CDSCO.
The import license fees vary by risk class; for Class A devices, it is approximately 50 per product.
Timeline and Processing Duration
| Step | Duration |
|---|---|
| Test License Application (MD13) | 1.5 - 2 months |
| Product Testing | 1 - 1.5 months |
| Document Preparation | Concurrent with testing |
| MD5 License Application (MD3) Submission | After test reports |
| Audit by Notified Body | 1 month |
| Query Resolution | 1 month |
| Total Time | 3 - 4 months |
Government Fees and Costs
- Test License (Form MD13): No separate fee but included in the overall process.
- MD5 License Application Fee: Rs. 5000 per application.
- Product Fee: Rs. 500 per product (for the Pupillometer).
- Additional costs may include testing laboratory fees (varies by lab), notified body audit fees (typically Rs. 50,000 - Rs. 1,00,000), and consultancy if engaged.
Common Challenges and Practical Solutions
- Delayed Product Testing: Scheduling with government labs can cause delays. To mitigate, initiate test license application early and consider private accredited labs where permissible.
- Incomplete Documentation: Missing or inconsistent details in Device Master File or Risk Management File often lead to queries. Employ experienced regulatory consultants to ensure completeness.
- Audit Non-Compliance: Manufacturers sometimes overlook quality system requirements. Implement ISO 13485:2016 standards and maintain audit readiness.
- Query Resolution Delays: Prompt and detailed responses to CDSCO queries expedite license issuance. Assign a dedicated regulatory liaison.
Expert Consultation and Support
With over 25 years of deep expertise and having supported 500+ companies, we specialize in end-to-end CDSCO licensing for ophthalmic devices like Pupillometers. Our tailored services include:
- Preparing and reviewing Device and Plant Master Files
- Coordinating product testing with CDSCO-approved labs
- Managing test license and manufacturing license applications
- Facilitating audit readiness and query resolution
- Offering up-to-date regulatory intelligence and compliance strategies
Getting Started with Your CDSCO License Application for Pupillometers
To embark on your licensing journey, follow these actionable steps:
Register on the CDSCO MD Online Portal: Begin by creating your account on the CDSCO MD Online Portal.
Apply for Test License (Form MD13): Submit your application early to initiate product testing.
Coordinate Product Testing: Engage with CDSCO-approved laboratories promptly; refer to the Testing Laboratories List.
Prepare Documentation: Utilize our Device Master File and Plant Master File guides to ensure compliance.
Plan for Audit: Identify the appropriate Notified Body and schedule the audit well in advance.
Submit Manufacturing License Application (Form MD3): Complete and submit on the CDSCO portal.
Prepare for Query Resolution: Assign a dedicated team member to handle prompt responses during the review phase.
By following this structured approach and leveraging our expertise, you can successfully obtain your CDSCO MD5 license for your Pupillometer and confidently enter the Indian ophthalmic device market.
For customized support or inquiries, feel free to reach out to our regulatory consulting team dedicated to medical device compliance in India.