CDSCO License for Sacculotomy tack (Cody tack)
Medical Device Information
Intended Use
Intended to be implanted to relieve the symptoms of vertigo.
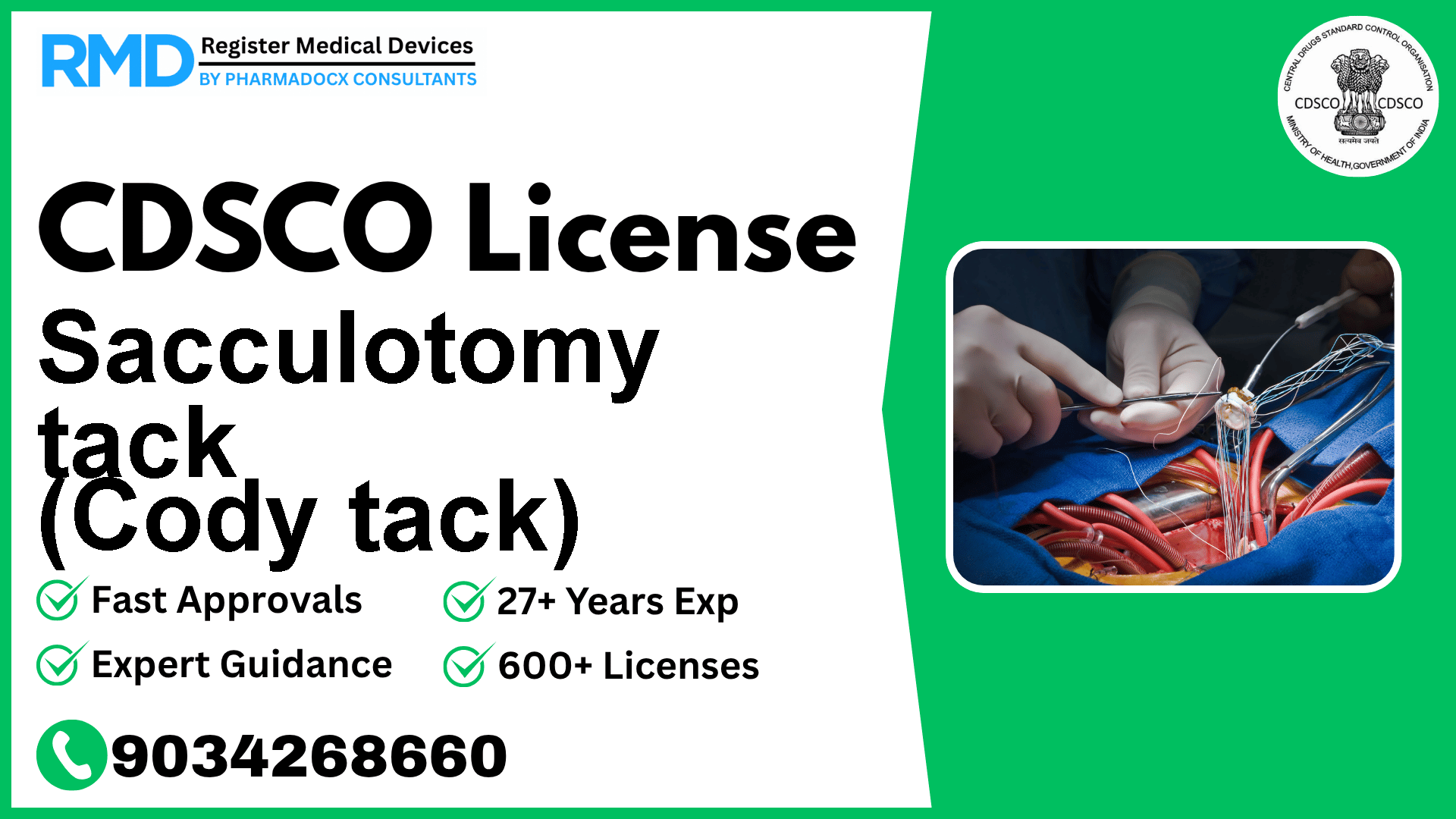
Comprehensive CDSCO Licensing Guide for Sacculotomy Tack (Cody Tack) - Class C Medical Device
As a device designed for internal prosthetic replacement to alleviate vertigo symptoms, the Sacculotomy Tack (Cody Tack) falls under Class C in the Indian medical device regulatory framework. With over 25 years of experience guiding 500+ companies through CDSCO licensing, we understand the nuances and critical steps to ensure a smooth approval process for this specialized device.
Understanding the Regulatory Importance of the Sacculotomy Tack
Implantable devices such as the Sacculotomy Tack require stringent regulatory oversight to ensure patient safety and compliance with Indian standards. The Central Drugs Standard Control Organization (CDSCO) classifies this device as Class C, indicating moderate to high risk due to its implantable nature and critical function. Compliance with CDSCO’s regulatory framework is mandatory before manufacturing or importing this device into India.
CDSCO Regulatory Framework for Internal Prosthetic Replacements (Class C Devices)
The CDSCO framework mandates that Class C devices undergo a centralized licensing process via the Central Licensing Authority. This includes obtaining an MD9 manufacturing license or MD15 import license, depending on whether you are manufacturing or importing the device. The process involves comprehensive product testing, documentation, audits, and compliance with Essential Principles for medical devices.
For detailed classification criteria, review the Medical Device Classification guide.
Risk Classification and License Requirements for Sacculotomy Tack
- Device: Sacculotomy Tack (Cody Tack)
- Risk Class: C (Moderate to High Risk)
- Category: Internal Prosthetic Replacement
- Regulatory Notification: 29/Misc/3/2017-DC (292) dated 06.06.2018
Given its Class C status, the device requires an MD9 manufacturing license from the CDSCO Central Licensing Authority and an MD15 import license for importers.
Manufacturing License Process for Class C Devices (MD9 License)
The MD9 license process typically spans 4-5 months. Here’s a structured overview:
Test License (Form MD13): Before applying for MD9, obtain a test license which takes approximately 1.5-2 months. This license allows you to manufacture devices for testing purposes.
Product Testing: Get the Sacculotomy Tack tested in government-approved testing laboratories to demonstrate compliance with safety and performance standards. Refer to the list of CDSCO-approved Testing Laboratories.
Document Preparation: Prepare comprehensive documentation including Device Master File, Plant Master File, Risk Management File, and Essential Principles Checklist.
Application Submission (Form MD7): Submit your MD9 license application through the CDSCO MD Online Portal.
Audit: CDSCO inspectors will conduct a detailed audit of your manufacturing site and quality systems.
Query Resolution: Address any queries raised by CDSCO during the review.
License Grant: Upon successful completion, the MD9 manufacturing license is issued.
For a detailed stepwise guide, visit our MD9 License Guide.
Manufacturing License Documents Required for Sacculotomy Tack
- Company Constitution (MOA and AOA)
- Proof of ownership or lease of manufacturing premises
- Documents of qualified technical staff (e.g., biomedical engineers, quality managers)
- Fire and Pollution NOC
- Device Master File (DMF) detailing design, materials, and specifications (Device Master File Guide)
- Plant Master File describing manufacturing processes and facilities (Plant Master File Guide)
- Essential Principles Checklist to demonstrate compliance
- Risk Management File highlighting hazard analysis and mitigation (Risk Management Resource)
- Test Reports from CDSCO-approved labs
- Device Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documentation (preferably ISO 13485:2016 certified)
Import License Process for Sacculotomy Tack (MD15 License)
For importers, the MD15 import license must be obtained from the CDSCO Central Licensing Authority. The process typically takes 5-6 months and involves:
Document Compilation: Prepare the required documentation including manufacturing license, Free Sale Certificate, ISO 13485:2016 certificate, CE Certificate, Device Master File, and Plant Master File.
Application Submission: Submit the application using Form MD14 via the CDSCO MD Online Portal.
Departmental Review and Query Resolution: CDSCO reviews the documents and may raise queries.
License Issuance: After satisfactory evaluation, the MD15 import license is granted.
For a practical walkthrough, see our Import License Guide.
Import License Documents Required for Sacculotomy Tack
- Valid Manufacturing License (MD9) from the country of origin
- Free Sale Certificate from the country of origin
- ISO 13485:2016 certificate
- CE Certificate (if applicable)
- Device Master File
- Plant Master File
- Wholesale License
- Company Constitution documents
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 to 2 months |
| Product Testing | 1 to 1.5 months |
| Document Preparation | Concurrent with testing |
| MD9 License Application | 4 to 5 months total (including audit and query resolution) |
| MD15 License Application (Import) | 5 to 6 months |
Government Fees and Costs
MD9 Manufacturing License Fees:
- Application Fee: ₹50,000
- Per Product Fee: ₹1,000 per product
Test License Fees:
- Usually nominal; check latest fee structure on CDSCO portal.
MD15 Import License Fees:
- For Class C devices like Sacculotomy Tack: ₹3,000 per site and ₹1,500 per product
All applications and fees are processed via the CDSCO MD Online Portal.
Common Challenges and Solutions
Delayed Testing Results: Government labs can have backlogs; plan testing early and consider private notified labs if available.
Incomplete Documentation: Missing files like Risk Management or Plant Master File delay approvals; leverage expert templates and checklists.
Audit Non-Compliance: Prepare for audits by conducting internal mock audits and addressing gaps proactively.
Query Management: Respond comprehensively and promptly to CDSCO queries to avoid unnecessary delays.
Expert Consultation and Support
Navigating the CDSCO licensing labyrinth for a Class C device like the Sacculotomy Tack demands expert knowledge. Our consultancy has successfully supported over 500 companies by providing tailored support—from documentation preparation to audit readiness and query resolution. We ensure your submissions are robust and compliant, minimizing time and cost.
Getting Started with Your CDSCO License Application
- Assess your device classification to confirm the need for an MD9 or MD15 license.
- Initiate a test license (MD13) application through the CDSCO MD Online Portal.
- Engage a CDSCO-approved testing laboratory early to schedule product tests.
- Compile your Device Master File and Plant Master File following best practices.
- Implement or update your Risk Management File in line with ISO standards.
- Prepare all supporting documents including QMS certifications, NOCs, and company constitutions.
- Submit your application online and prepare for the audit.
Our team is ready to assist you every step of the way. Contact us to schedule a consultation and kickstart your licensing journey with confidence.
Unlock the Indian market potential for your Sacculotomy Tack with a compliant, expertly managed CDSCO licensing process today.