CDSCO License for Scotometer
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
An instrument used for the recording and measuring of the areas of field of vision that is reduced, i.e., relative scotoma, or loss of sensitivity to light (absolute scotoma or blind spots).
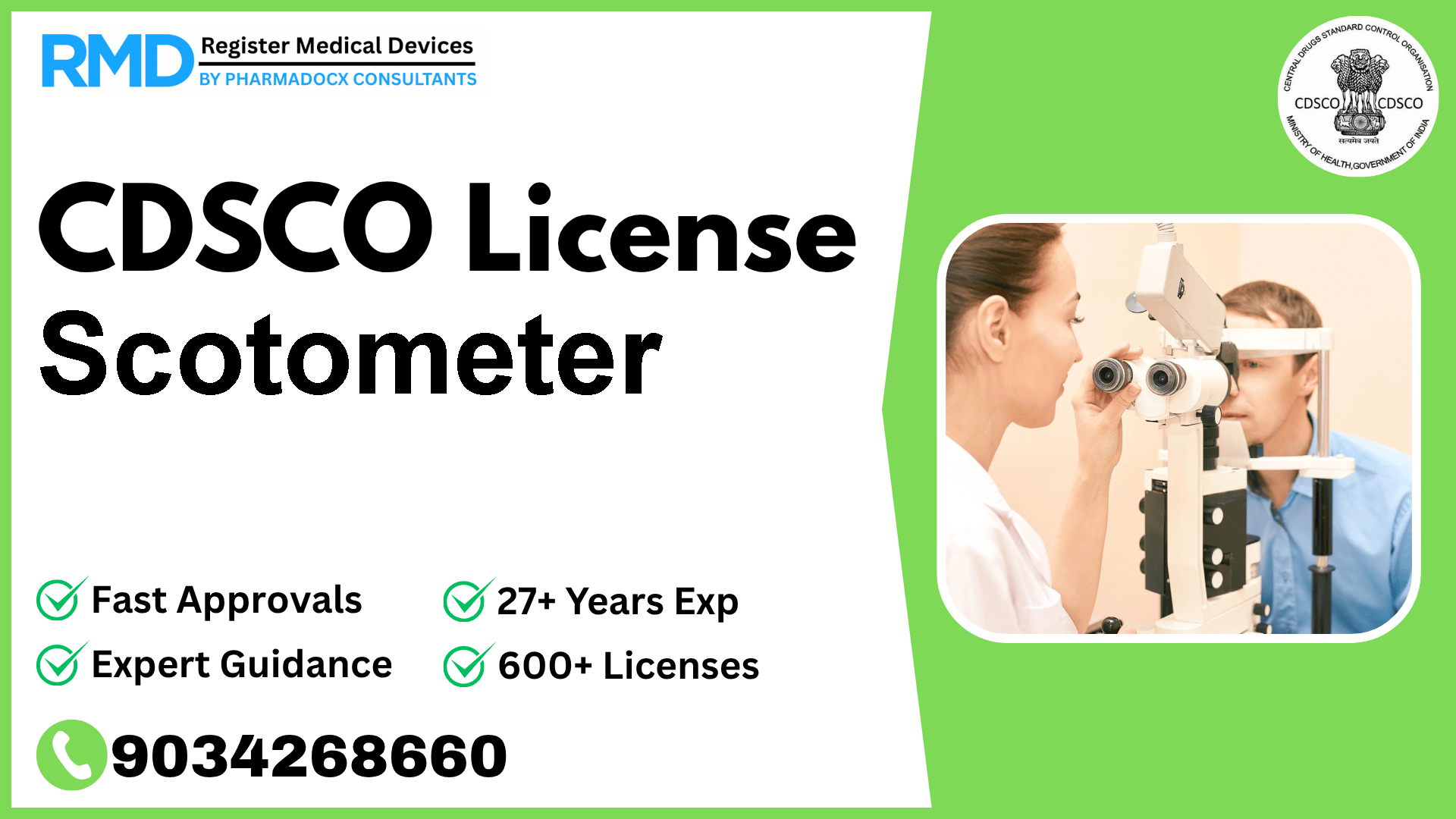
Understanding the Scotometer and Its Regulatory Importance in India
The Scotometer is a specialized ophthalmic instrument designed to record and measure the areas of the visual field where sensitivity to light is reduced or absent—commonly referred to as relative or absolute scotomas (blind spots). Given its critical role in diagnosing and managing eye disorders, regulatory compliance under the Central Drugs Standard Control Organization (CDSCO) is mandatory before manufacturing or marketing the Scotometer in India.
At our consultancy, with over 25 years of experience and having assisted 500+ companies, we emphasize the importance of understanding the regulatory framework to ensure a smooth entry into the Indian medical device market.
CDSCO Regulatory Framework for Scotometer (Class A Medical Device)
The CDSCO classifies medical devices into four risk categories: Class A (low risk), Class B (low-moderate risk), Class C (moderate-high risk), and Class D (high risk). The Scotometer falls under Class A, the lowest risk category, but still requires strict adherence to regulatory norms.
This device is notified under the Gazette Notification Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021, which governs ophthalmology devices including the Scotometer.
Manufacturers of Class A devices must obtain a Manufacturing License (MD5) from the State Licensing Authority before production and sale. Importers require a separate import license (MD15), but this guide primarily focuses on manufacturers.
Risk Classification and License Requirements for Scotometer
- Risk Class: A (Low Risk)
- License Type: MD5 Manufacturing License
- Governing Authority: State Licensing Authority
- Application Form: MD3 for license application; MD13 for test license
The MD5 license process includes obtaining a test license initially, mandatory product testing, document submission, and audit by a notified body.
Step-by-Step Manufacturing License Process for Scotometer (MD5 License)
1. Obtain Test License (Form MD13)
Before applying for the manufacturing license, you must secure a test license to manufacture and test the Scotometer. This process takes approximately 1.5 to 2 months.
2. Product Testing
The Scotometer must be tested at CDSCO-approved laboratories to verify compliance with Indian standards. Tests typically cover electrical safety, performance, and biocompatibility.
You can find the approved labs on the CDSCO Testing Laboratories list.
3. Document Preparation
Prepare comprehensive documentation including Device Master File (DMF), Plant Master File (PMF), risk management reports, and other essential documents. Detailed templates and explanations can be found in our Device Master File guide and Plant Master File guide.
4. Submit Manufacturing License Application (Form MD3)
Apply through the CDSCO MD Online Portal with all required documents.
5. Audit by Notified Body
An audit will be conducted by a notified body from the official List of Notified Bodies to verify compliance with quality standards and manufacturing practices.
6. Address Queries and Obtain License
Respond promptly to any queries raised by the CDSCO or the notified body. Upon satisfactory completion, the MD5 license will be granted on Form MD5.
Comprehensive Documentation Required for Scotometer MD5 License
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Qualification and Experience Certificates of Technical Staff
- Fire Safety NOC
- Pollution Control Board NOC
- Device Master File (DMF) including detailed specifications and design
- Plant Master File (PMF) detailing manufacturing environment and equipment
- Essential Principles Compliance Checklist
- Risk Management File complying with ISO 14971 standards
- Test Reports from CDSCO-approved laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) Documents, preferably ISO 13485 certified
Timeline and Processing Duration
| Process Step | Estimated Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 3 - 4 weeks |
| Document Preparation | 3 - 4 weeks |
| License Application Submission | Immediate |
| Audit by Notified Body | 4 - 6 weeks |
| Query Resolution and Grant | 3 - 4 weeks |
| Total Estimated Time | 3 to 4 months |
Government Fees and Costs
For the Scotometer, as a Class A device, the fees are as follows:
- Application Fee: Rs. 5,000 per application
- Product Fee: Rs. 500 per product (Scotometer considered as one product)
Additional costs to consider:
- Testing laboratory fees (varies by test complexity)
- Notified body audit charges (varies but typically Rs. 50,000 to Rs. 1,00,000)
- Consultancy fees if availing expert help
Common Challenges and Practical Solutions
Challenge 1: Delays in Test License Processing
Solution: Submit a complete and error-free application with all required supporting documents to avoid back-and-forth queries.
Challenge 2: Difficulty in Product Testing
Solution: Early engagement with CDSCO-approved testing labs and pre-assessment of product to ensure compliance with Indian standards.
Challenge 3: Audit Non-Conformities
Solution: Prepare a robust QMS and documentation ahead of the audit. Conduct internal mock audits to identify gaps.
Challenge 4: Incomplete Risk Management Documentation
Solution: Implement ISO 14971 based risk management practices and maintain detailed risk analysis and mitigation records. Our Risk Management guide offers valuable insights.
Expert Consultation and Support
Navigating CDSCO licensing can be complex, especially for precision devices like the Scotometer. Our team has successfully guided 500+ medical device manufacturers through the MD5 licensing process, ensuring compliance and timely approvals.
We provide end-to-end support including:
- Gap analysis and documentation assistance
- Vendor coordination for testing and audits
- Application filing and follow-ups
- Regulatory intelligence updates
Getting Started with Your Scotometer CDSCO License Application
- Evaluate Your Current Compliance Status: Conduct an internal audit of your manufacturing setup, QMS, and documentation.
- Initiate Test License Application: Prepare and submit Form MD13 with required documents through the CDSCO MD Online Portal.
- Engage Approved Testing Labs: Schedule product testing early to avoid delays.
- Prepare Comprehensive Documentation: Use our Device Master File guide and Plant Master File guide to assemble robust files.
- Plan for Notified Body Audit: Select a notified body from the CDSCO List and prepare for inspection.
- Submit MD5 License Application: Once test license and testing are complete, file Form MD3 with all supporting documentation.
By following these practical steps and leveraging expert assistance, manufacturers can efficiently obtain their CDSCO MD5 license for the Scotometer and confidently enter the Indian ophthalmology device market.
For tailored support and to discuss your specific requirements, feel free to contact our regulatory consulting team.