CDSCO License for Septostomy Catheter
Medical Device Information
Intended Use
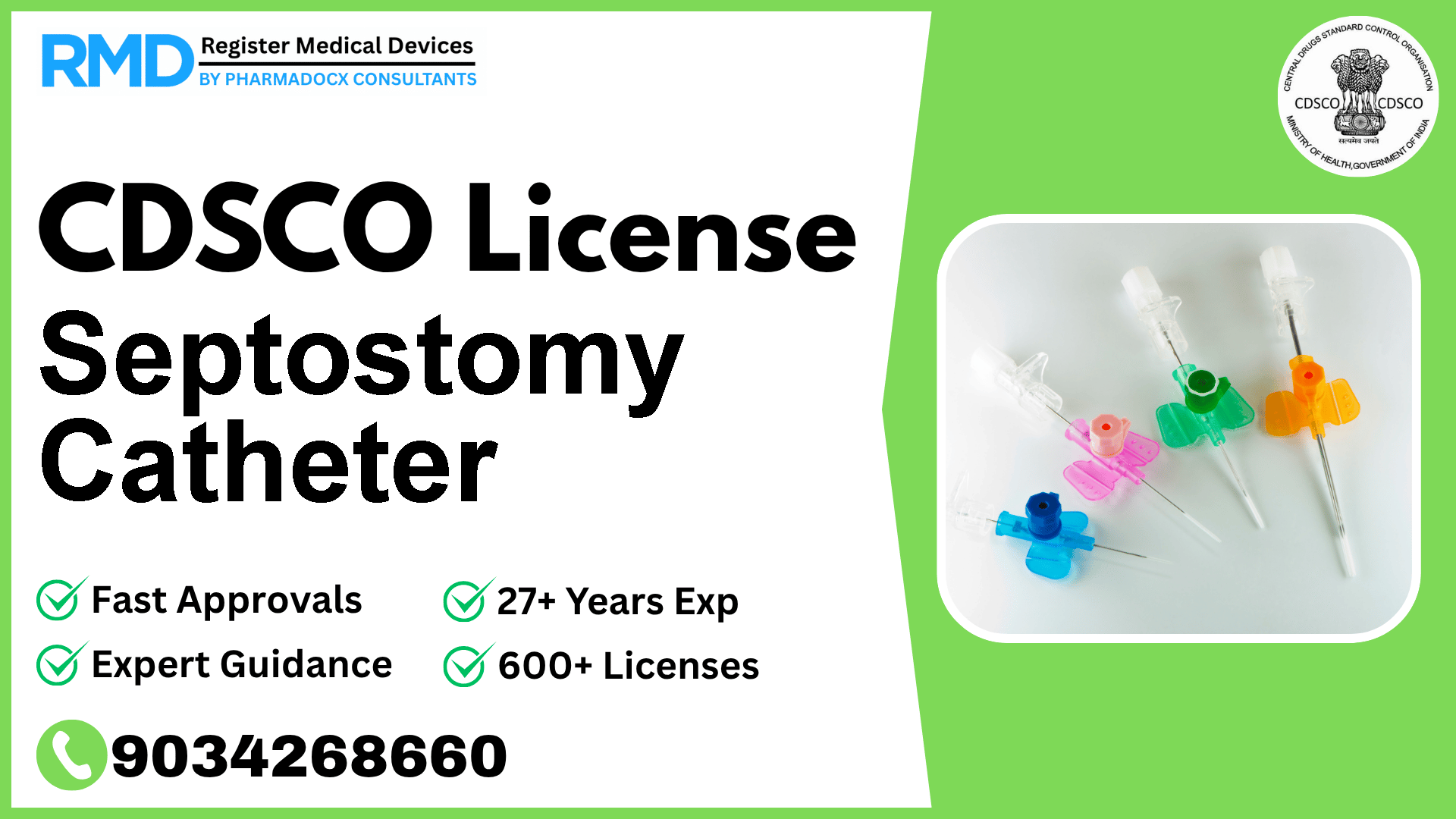
Used to enlarge interatrial openings
Comprehensive Guide to CDSCO Licensing for Septostomy Catheters (Class D Medical Device)
At [Our Company], with over 25 years of experience and having supported 500+ clients in medical device regulatory approvals, we understand the critical importance of securing a CDSCO license for your Class D medical device such as the Septostomy Catheter. This device, used to enlarge interatrial openings, falls under the highest risk category, necessitating stringent compliance and a thorough understanding of the regulatory framework.
CDSCO Regulatory Framework for Septostomy Catheters
Septostomy Catheters are regulated under the Indian Medical Device Rules and require adherence to the Central Drugs Standard Control Organization (CDSCO) guidelines. As a Class D device, it is subject to rigorous evaluation to ensure safety and efficacy before entering the Indian market.
Risk Classification and License Requirements
According to Medical Device Classification, Septostomy Catheters are classified as Class D due to their invasive cardiac application.
- License Type: MD9 Manufacturing License
- Authority: Central Licensing Authority (CDSCO, New Delhi)
- Application Form: MD7
Manufacturing License Process for Class D Devices (MD9 License)
The MD9 license process involves several key steps:
- Test License (Form MD13): Initially, you must obtain a test license, which typically takes 1.5 to 2 months. This allows for product testing in government-approved labs.
- Product Testing: Testing must be conducted at CDSCO-recognized laboratories; a list can be found on the Testing Laboratories page.
- Document Preparation: Compile comprehensive documentation including Device Master File, Plant Master File, Risk Management File, and QMS documents.
- License Application: Submit Form MD7 through the CDSCO MD Online Portal.
- Audit: CDSCO inspectors conduct an on-site audit to verify compliance.
- Query Resolution: Address any queries raised during audit or document review.
- Grant of License: Upon satisfactory compliance, the license is issued on Form MD9.
For more detailed guidance, refer to our MD9 License Guide.
Manufacturing License Documents Required for Septostomy Catheters
Ensure your application includes:
- Company Constitution documents
- Proof of ownership or lease of manufacturing premises
- Details and qualifications of technical staff
- Fire Safety NOC
- Pollution Control Board NOC
- Device Master File (DMF) - see our DMF Guide
- Plant Master File (PMF) - refer to our Plant Master File Guide
- Essential Principles Checklist specific to Septostomy Catheter
- Risk Management File complying with ISO 14971 (Risk Management Guide)
- Test Reports from accredited labs
- Product labels and Instructions for Use (IFU)
- Quality Management System (QMS) documentation, typically ISO 13485:2016 certification
Import License Process (MD15) for Septostomy Catheters
If you plan to import Septostomy Catheters:
- License Type: MD15 Import License
- Application Form: MD14
- Process: Document preparation → Application submission → Query resolution → License grant
- Documents Required: Manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device and Plant Master Files, Wholesale License, Company Constitution
Fees vary by risk class; for Class D devices, expect approximately 1500 per product.
More details are available in our Import License Guide.
Timeline and Processing Duration
For Class D Septostomy Catheters, expect the following approximate timelines:
- Test License (MD13): 1.5 - 2 months
- Testing: 1 - 1.5 months depending on lab backlog
- Document Preparation: Ongoing; can be done parallelly
- MD9 License Processing: 2 - 2.5 months
Total Duration: 4 - 5 months from start to grant of MD9 license.
Government Fees and Costs
- MD9 Application Fee: Rs. 50,000 per application
- Per Product Fee: Rs. 1,000
- Testing and Audit Costs: Additional, varies by lab and notified body
Budgeting for these fees early can prevent application delays.
Common Challenges and Solutions
- Incomplete Documentation: Prepare your Device Master File and Risk Management documentation meticulously. Utilize our guides to avoid omissions.
- Audit Non-compliance: Engage with notified bodies early; check the Notified Bodies List for audit requirements.
- Testing Delays: Book slots at accredited labs well in advance.
- Query Response Delays: Assign a dedicated regulatory expert to manage queries promptly.
Expert Consultation and Support
Navigating CDSCO licensing for Class D devices like the Septostomy Catheter can be complex. Our team has successfully guided over 500 companies through this process, ensuring timely approvals and compliance with all regulatory norms.
We provide:
- End-to-end documentation support
- Liaison with CDSCO and notified bodies
- Pre-audit readiness assessments
- Post-approval compliance monitoring
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm Class D status using the Medical Device Classification tool.
- Initiate Test License Application: Prepare and submit Form MD13 via the CDSCO MD Online Portal.
- Engage Accredited Testing Labs: Arrange product testing early to align with your application timeline.
- Prepare Comprehensive Documentation: Start compiling the Device Master File, Plant Master File, Risk Management File, and QMS documents.
- Plan for Audit: Schedule and prepare for the mandatory CDSCO inspection.
- Submit MD9 Application: Once test license and testing are complete, apply using Form MD7.
Our expert team is ready to guide you through each step to ensure your Septostomy Catheter gains timely CDSCO approval, enabling your successful entry into the Indian medical device market.