CDSCO License for X-ray radiation therapy system
Medical Device Information
Intended Use
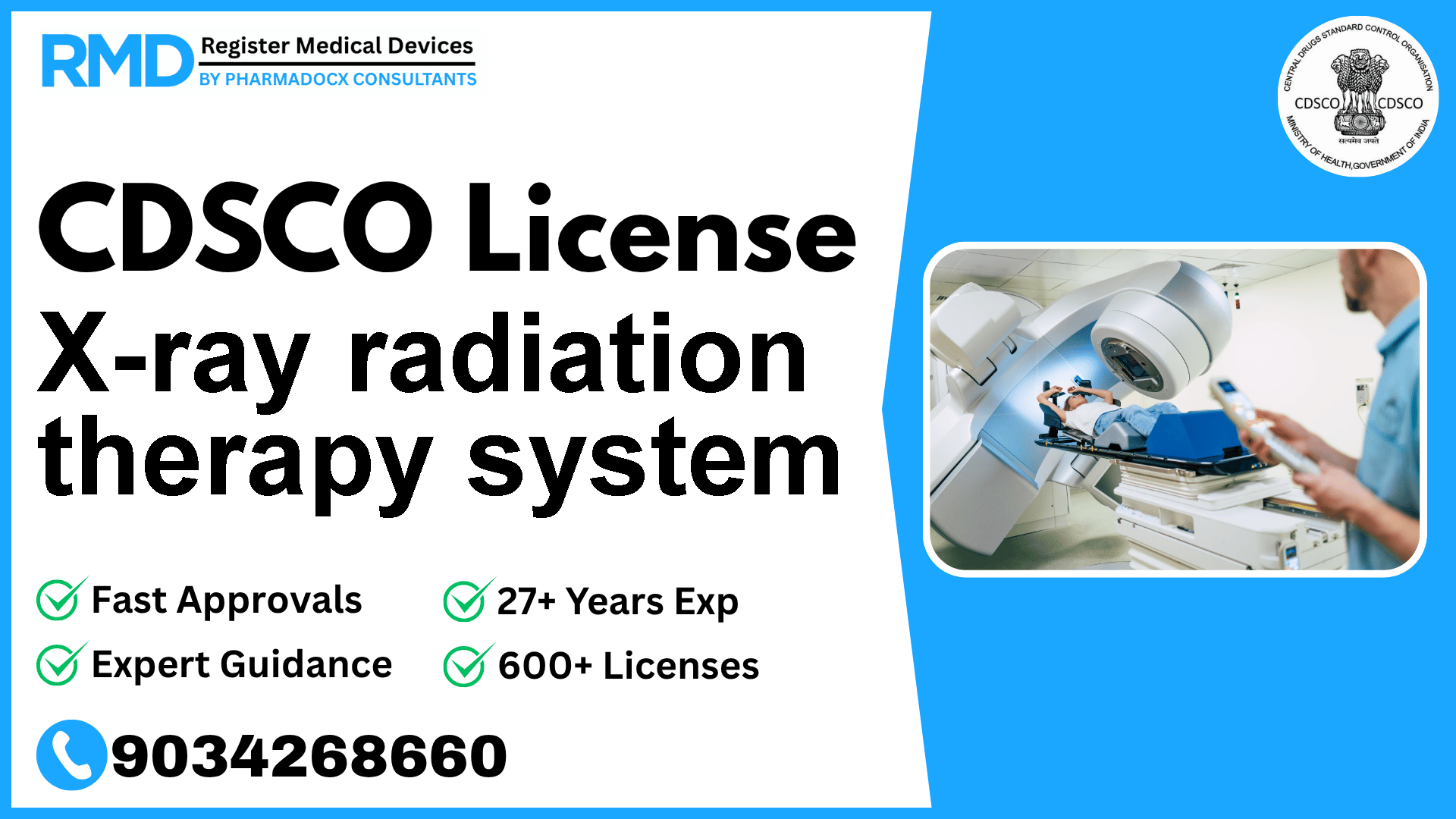
An x-ray radiation therapy system is a device intended to produce and control x-rays used for radiation therapy.
Comprehensive Guide to CDSCO Licensing for X-ray Radiation Therapy Systems (Class C Medical Devices)
With over 25 years of regulatory experience and having supported more than 500 medical device companies, we understand the complexities involved in navigating India's CDSCO licensing framework for high-risk devices such as X-ray radiation therapy systems. These devices, classified as Class C under the CDSCO regulations, require meticulous preparation, thorough testing, and strict compliance to enter the Indian market.
Understanding the X-ray Radiation Therapy System and Its Regulatory Importance
An X-ray radiation therapy system is a sophisticated medical device designed to generate and precisely control X-rays for therapeutic applications, primarily cancer treatment. Given its critical role and potential risks associated with ionizing radiation exposure, the device falls under Class C risk category, necessitating stringent regulatory oversight by the Central Drugs Standard Control Organization (CDSCO).
The device is notified under the Medical Device Rules (MDR) via File No. 29/Misc./03/2020-DC (180) dated 6.8.2021, which formalizes its classification and regulatory requirements. Compliance with CDSCO ensures patient safety, efficacy of the device, and legal market access in India.
CDSCO Regulatory Framework for X-ray Radiation Therapy Systems
The CDSCO regulates medical devices based on risk classification:
- Class A and B: Low-risk devices, licensed by State Authorities
- Class C and D: Medium to high-risk devices, licensed by Central Authorities
As a Class C device, the X-ray radiation therapy system mandates a centralized licensing process involving:
- Manufacturing License: MD9 license (Form MD7)
- Import License: MD15 license (Form MD14)
Both licenses require comprehensive documentation, product testing from government-approved laboratories, and audits by CDSCO inspectors.
Risk Classification and License Requirements for X-ray Radiation Therapy Systems
Class C devices are considered moderate to high risk due to their direct impact on critical patient health parameters. The CDSCO mandates:
- MD9 Manufacturing License: For Indian manufacturers
- MD15 Import License: For importing entities
The key regulatory steps include obtaining a test license (MD13), product testing, audit, and final license grant.
Manufacturing License Process (MD9) for X-ray Radiation Therapy Systems
The MD9 license process is managed by the Central Licensing Authority and involves the following stages:
- Test License Application (Form MD13): Required before manufacturing to enable product testing. Processing time is approximately 1.5–2 months.
- Product Testing: Conducted at CDSCO-approved testing laboratories. For radiotherapy devices, testing includes electrical safety, radiation emission compliance, and performance validation.
- Document Preparation: Assemble all required technical and quality management documents.
- MD9 License Application (Form MD7): Submit via the CDSCO MD Online Portal.
- Audit by CDSCO Inspectors: On-site inspection of manufacturing facilities and quality systems.
- Query Resolution: Address any observations or queries raised during audit.
- Grant of License (Form MD9): Upon satisfactory compliance.
The entire process typically spans 4–5 months.
Manufacturing License Documents Required
For an X-ray radiation therapy system, the documentation must be robust and include:
- Company constitution and incorporation certificates
- Proof of ownership or lease of manufacturing premises
- Technical staff qualifications and experience
- Fire NOC and pollution control NOC
- Device Master File (DMF) detailing design, specifications, and manufacturing process (Device Master File Guide)
- Plant Master File (PMF) reflecting facility and equipment details (Plant Master File Guide)
- Essential Principles Checklist confirming compliance with safety and performance standards
- Risk Management File documenting hazard analysis and mitigation (Risk Management)
- Product test reports from government-approved laboratories (Testing Laboratories)
- Labels, packaging, and Instructions for Use (IFU) compliant with CDSCO guidelines
- Quality Management System documents, typically ISO 13485:2016 certification
Import License Process (MD15) for X-ray Radiation Therapy Systems
Importers must secure the MD15 license from CDSCO’s Central Licensing Authority. The process involves:
- Document Preparation: Similar to manufacturing but must also include import-specific documents.
- Application Submission: Use Form MD14 on the CDSCO MD Online Portal.
- Review and Query Resolution: CDSCO reviews documentation and may request clarifications.
- Grant of MD15 License: On approval.
Unlike the manufacturing process, no test license is required for importers. The typical timeline is 5–6 months.
Import License Documents Required
Key documents required for the MD15 license include:
- Valid manufacturing license from the country of origin
- Free Sale Certificate or equivalent from the exporting country
- ISO 13485:2016 certification
- CE Certificate or other internationally recognized conformity marks
- Device Master File and Plant Master File
- Wholesale license (if applicable)
- Company constitution and incorporation documents
- Detailed product dossier including labeling and IFU
Timeline and Processing Duration
| License Type | Process Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| MD9 Manufacturing | 4 – 5 months total (includes test license and audit) |
| MD15 Import | 5 – 6 months |
Planning ahead is critical. For example, initiating the test license early can prevent delays.
Government Fees and Costs
For an X-ray radiation therapy system (Class C), the fees are as follows:
- MD9 Manufacturing License: ₹50,000 per application + ₹1,000 per product
- MD15 Import License:
- ₹3,000 per site
- ₹1,500 per product
Additional costs include testing fees at government-approved laboratories, potential consultancy fees, and audit-related expenses.
Common Challenges and Solutions
1. Delay in Product Testing: Testing labs may have backlogs. To mitigate, pre-book testing slots early and ensure test samples are prepared per lab specifications.
2. Incomplete Documentation: Many applicants overlook detailed risk management files or Device Master Files. Utilize comprehensive checklists and expert reviews before submission.
3. Audit Non-compliance: Audits can be rigorous. Conduct internal pre-audit assessments and ensure that QMS and facility conditions strictly meet CDSCO expectations.
4. Query Resolution Delays: Respond promptly and thoroughly to CDSCO queries. Engage regulatory consultants to draft precise responses.
Expert Consultation and Support
Navigating the MD9 and MD15 licensing pathways requires specialized knowledge. Our 25+ years of experience enable us to streamline applications, prepare detailed documentation, coordinate with notified bodies and testing labs, and provide audit support. Leveraging our expertise reduces approval timelines and avoids common pitfalls.
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm your device under the CDSCO Medical Device Rules (Medical Device Classification).
- Prepare Essential Files: Develop Device Master File, Plant Master File, and Risk Management File.
- Apply for Test License (MD13): Submit through the CDSCO MD Online Portal to start product testing.
- Coordinate Testing: Engage with approved government testing laboratories early (Testing Laboratories).
- Compile Audit-Ready Documentation: Align your QMS and manufacturing site with CDSCO standards.
- Submit MD9 or MD15 Application: Depending on your role as manufacturer or importer.
- Prepare for Audit & Queries: Stay responsive and ready for site inspections.
Starting early and seeking expert guidance ensures a smoother pathway to market entry for your X-ray radiation therapy system. Contact us for tailored consulting to successfully obtain your CDSCO licenses.